Systematic review and network meta-analysis of the efficacy of existing treatments for patients with recurrent glioblastoma
- PMID: 34095835
- PMCID: PMC8174573
- DOI: 10.1093/noajnl/vdab052
Systematic review and network meta-analysis of the efficacy of existing treatments for patients with recurrent glioblastoma
Abstract
Background: Despite advances in the treatment of cancers over the last years, treatment options for patients with recurrent glioblastoma (rGBM) remain limited with poor outcomes. Many regimens have been investigated in clinical trials; however, there is a lack of knowledge on comparative effectiveness. The aim of this systematic review is to provide an overview of existing treatment strategies and to estimate the relative efficacy of these regimens in terms of progression-free survival (PFS) and overall survival (OS).
Methods: We conducted a systematic review to identify randomized controlled trials (RCTs) investigating any treatment regimen in adult patients suffering from rGBM. Connected studies reporting at least one of our primary outcomes were included in a Bayesian network meta-analysis (NMA) estimating relative treatment effects.
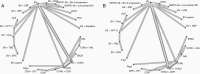
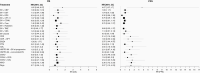
Results: Forty RCTs fulfilled our inclusion criteria evaluating the efficacy of 38 drugs as mono- or combination therapy. Median OS ranged from 2.9 to 18.3 months; median PFS ranged from 0.7 to 6 months. We performed an NMA including 24 treatments that were connected within a large evidence network. Our NMA indicated improvement in PFS with most bevacizumab (BV)-based regimens compared to other regimens. We did not find any differences in OS between treatments.
Conclusion: This systematic review provides a comprehensive overview of existing treatment options for rGBM. The NMA provides relative effects for many of these treatment regimens, which have not been directly compared in RCTs. Overall, outcomes for patients with rGBM remain poor across all treatment options, highlighting the need for innovative treatment options.
Keywords: network meta-analysis; overall survival; progression-free survival; recurrent glioblastoma; systematic review.
© The Author(s) 2021. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures





Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Bevacizumab for recurrent glioblastoma: a systematic review and meta-analysis.Eur Rev Med Pharmacol Sci. 2021 Nov;25(21):6480-6491. doi: 10.26355/eurrev_202111_27092. Eur Rev Med Pharmacol Sci. 2021. PMID: 34787852
-
Lessons learned from contemporary glioblastoma randomized clinical trials through systematic review and network meta-analysis: part 2 recurrent glioblastoma.Neurooncol Adv. 2021 Feb 12;3(1):vdab029. doi: 10.1093/noajnl/vdab029. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 34042101 Free PMC article. Review.
-
A Bayesian Network Meta-Analysis for Identifying the Optimal Taxane-Based Chemotherapy Regimens for Treating Gastric Cancer.Front Pharmacol. 2019 Jul 5;10:717. doi: 10.3389/fphar.2019.00717. eCollection 2019. Front Pharmacol. 2019. PMID: 31333452 Free PMC article.
-
Comparative efficacy and safety of anti-vascular endothelial growth factor regimens for neovascular age-related macular degeneration: systematic review and Bayesian network meta-analysis.Ther Adv Chronic Dis. 2020 Sep 4;11:2040622320953349. doi: 10.1177/2040622320953349. eCollection 2020. Ther Adv Chronic Dis. 2020. PMID: 32953000 Free PMC article.
Cited by
-
Spatial Distribution of Immune Cells in Primary and Recurrent Glioblastoma: A Small Case Study.Cancers (Basel). 2023 Jun 20;15(12):3256. doi: 10.3390/cancers15123256. Cancers (Basel). 2023. PMID: 37370866 Free PMC article.
-
Identifying the best treatment choice for relapsing/refractory glioblastoma: a systematic review with multiple Bayesian network meta-analyses.Oncologist. 2025 Jul 4;30(7):oyae338. doi: 10.1093/oncolo/oyae338. Oncologist. 2025. PMID: 39674575 Free PMC article.
-
Recent advances and future challenges of tumor vaccination therapy for recurrent glioblastoma.Cell Commun Signal. 2023 Apr 12;21(1):74. doi: 10.1186/s12964-023-01098-0. Cell Commun Signal. 2023. PMID: 37046332 Free PMC article. Review.
-
A Sequential Targeting Strategy Interrupts AKT-Driven Subclone-Mediated Progression in Glioblastoma.Clin Cancer Res. 2023 Jan 17;29(2):488-500. doi: 10.1158/1078-0432.CCR-22-0611. Clin Cancer Res. 2023. PMID: 36239995 Free PMC article.
-
Cross-reactivity between histone demethylase inhibitor valproic acid and DNA methylation in glioblastoma cell lines.Front Oncol. 2022 Nov 16;12:1033035. doi: 10.3389/fonc.2022.1033035. eCollection 2022. Front Oncol. 2022. PMID: 36465345 Free PMC article.
References
-
- Qazi MA, Vora P, Venugopal C, et al. . Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol. 2017;28(7):1448–1456. - PubMed
-
- Franceschi E, Bartolotti M, Brandes AA. Bevacizumab in recurrent glioblastoma: open issues. Future Oncol. 2015;11(19):2655–2665. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
