Indole - a promising pharmacophore in recent antiviral drug discovery
- PMID: 34095843
- PMCID: PMC8126882
- DOI: 10.1039/d0md00288g
Indole - a promising pharmacophore in recent antiviral drug discovery
Abstract
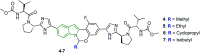
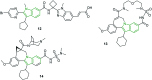
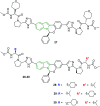
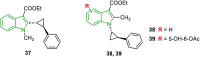
The bicyclic molecule indole has been in the limelight because of its numerous pharmacological potencies. It is used as an excellent scaffold in drug discovery of medicinal drugs such as antimicrobials, anticancer agents, antihypertensives, anti-proliferative agents and anti-inflammatory agents. In spite of its diverse therapeutic activity, it is used as a key pharmacophore in synthesizing the most potent biological agents. Besides, viral infections are ubiquitous and their prevention and cure have become a great challenge. In this regard, the design of indole-containing antiviral drugs is accomplished to combat viral infections. A lot of research is being carried out towards antiviral drug discovery by many researchers round the clock. Herein, the antiviral activity of recently discovered indole scaffolds is compiled and critically evaluated to give a meaningful summary. In addition, the structure-activity relationship of remarkable antiviral agents is discussed. Also, the structural motifs attributed to noteworthy antiviral properties are highlighted to guide future antiviral research.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The author declares there is no potential conflict of interest.
Figures








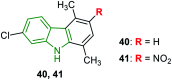


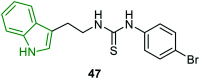

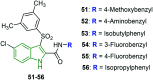
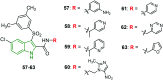







Similar articles
-
A Review of the Therapeutic Importance of Indole Scaffold in Drug Discovery.Curr Drug Discov Technol. 2023;20(6):9-37. doi: 10.2174/1570163820666230505120553. Curr Drug Discov Technol. 2023. PMID: 37151073
-
Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives.Bioorg Chem. 2019 Aug;89:103021. doi: 10.1016/j.bioorg.2019.103021. Epub 2019 May 30. Bioorg Chem. 2019. PMID: 31176854 Review.
-
An insight into the recent developments in anti-infective potential of indole and associated hybrids.J Mol Struct. 2022 Aug 5;1261:132808. doi: 10.1016/j.molstruc.2022.132808. Epub 2022 Mar 11. J Mol Struct. 2022. PMID: 35291692 Free PMC article. Review.
-
Phthalimide as a versatile pharmacophore scaffold: Unlocking its diverse biological activities.Drug Dev Res. 2023 Nov;84(7):1346-1375. doi: 10.1002/ddr.22094. Epub 2023 Jul 26. Drug Dev Res. 2023. PMID: 37492986 Review.
-
Screening of indole derivatives as the potent anticancer agents on dihydrofolate reductase: pharmaco-informatics and molecular dynamics simulation.J Biomol Struct Dyn. 2023 May;41(8):3667-3679. doi: 10.1080/07391102.2022.2053745. Epub 2022 Mar 23. J Biomol Struct Dyn. 2023. PMID: 35318890
Cited by
-
Indole-Containing Metal Complexes and Their Medicinal Applications.Molecules. 2024 Jan 18;29(2):484. doi: 10.3390/molecules29020484. Molecules. 2024. PMID: 38257397 Free PMC article. Review.
-
Regiochemical Effects on the Carbohydrate Binding and Selectivity of Flexible Synthetic Carbohydrate Receptors with Indole and Quinoline Heterocyclic Groups.European J Org Chem. 2021 Oct 7;2021(37):5262-5274. doi: 10.1002/ejoc.202100763. Epub 2021 Sep 12. European J Org Chem. 2021. PMID: 35694139 Free PMC article.
-
Antiviral Activity of an Indole-Type Compound Derived from Natural Products, Identified by Virtual Screening by Interaction on Dengue Virus NS5 Protein.Viruses. 2023 Jul 17;15(7):1563. doi: 10.3390/v15071563. Viruses. 2023. PMID: 37515249 Free PMC article.
-
Controllable 1,3-Bis-Functionalization of 2-Nitroglycals with High Regioselectivity and Stereoselectivity Enabled by a H-Bond Catalyst.JACS Au. 2024 Mar 11;4(3):974-984. doi: 10.1021/jacsau.3c00727. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559736 Free PMC article.
-
A multicomponent reaction for modular assembly of indole-fused heterocycles.Chem Sci. 2024 Mar 4;15(14):5211-5217. doi: 10.1039/d4sc00522h. eCollection 2024 Apr 3. Chem Sci. 2024. PMID: 38577354 Free PMC article.
References
-
- Kanwal A. Ahmad M. Aslam S. Naqvi S. A. R. Saif M. J. Pharm. Chem. J. 2019;53:179–187. doi: 10.1007/s11094-019-01976-3. - DOI
-
- Osman H. Yusufzai S. K. Khan M. S. Abd Razik B. M. Sulaiman O. Mohamad S. Gansau J. A. Ezzat M. O. Parumasivam T. Hassan M. Z. J. Mol. Struct. 2018;1166:147–154. doi: 10.1016/j.molstruc.2018.04.031. - DOI
Publication types
LinkOut - more resources
Full Text Sources

