Vaccine-induced ICOS+CD38+ circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways
- PMID: 34095875
- PMCID: PMC8149371
- DOI: 10.1016/j.xcrm.2021.100262
Vaccine-induced ICOS+CD38+ circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways
Abstract
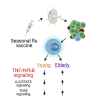
Humoral immune responses are dysregulated with aging, but the cellular and molecular pathways involved remain incompletely understood. In particular, little is known about the effects of aging on T follicular helper (Tfh) CD4 cells, the key cells that provide help to B cells for effective humoral immunity. We performed transcriptional profiling and cellular analysis on circulating Tfh before and after influenza vaccination in young and elderly adults. First, whole-blood transcriptional profiling shows that ICOS+CD38+ cTfh following vaccination preferentially enriches in gene sets associated with youth versus aging compared to other circulating T cell types. Second, vaccine-induced ICOS+CD38+ cTfh from the elderly had increased the expression of genes associated with inflammation, including tumor necrosis factor-nuclear factor κB (TNF-NF-κB) pathway activation. Finally, vaccine-induced ICOS+CD38+ cTfh display strong enrichment for signatures of underlying age-associated biological changes. These data highlight the ability to use vaccine-induced cTfh as cellular "biosensors" of underlying inflammatory and/or overall immune health.
Keywords: CD4; NF-kB; T follicular helper; aging; cellular biosensors; influenza; network analysis; vaccine.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests. E.J.W. consults or is an advisor for Merck, Elstar, Janssen, Related Sciences, Synthekine, and Surface Oncology, unrelated to the present study. E.J.W. is a founder of Surface Oncology and Arsenal Biosciences, unrelated to the present study. E.J.W. is an inventor on a patent (US patent no. 10,370,446) submitted by Emory University that covers the use of PD-1 blockade to treat infections and cancer.
Figures





Similar articles
-
Dysfunctional peripheral T follicular helper cells dominate in people with impaired influenza vaccine responses: Results from the FLORAH study.PLoS Biol. 2019 May 17;17(5):e3000257. doi: 10.1371/journal.pbio.3000257. eCollection 2019 May. PLoS Biol. 2019. PMID: 31100059 Free PMC article.
-
Production of IgG2 Antibodies to Pneumococcal Polysaccharides After Vaccination of Treated HIV Patients May Be Augmented by IL-7Rα Signaling in ICOS+ Circulating T Follicular-Helper Cells.Front Immunol. 2019 Apr 24;10:839. doi: 10.3389/fimmu.2019.00839. eCollection 2019. Front Immunol. 2019. PMID: 31068934 Free PMC article.
-
ICOS signaling promotes a secondary humoral response after re-challenge with Plasmodium chabaudi chabaudi AS.PLoS Pathog. 2020 Apr 29;16(4):e1008527. doi: 10.1371/journal.ppat.1008527. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32348365 Free PMC article.
-
Inducible T-cell co-stimulator: Signaling mechanisms in T follicular helper cells and beyond.Immunol Rev. 2019 Sep;291(1):91-103. doi: 10.1111/imr.12771. Immunol Rev. 2019. PMID: 31402504 Review.
-
T follicular helper cells in human autoimmunity.Curr Opin Immunol. 2016 Dec;43:24-31. doi: 10.1016/j.coi.2016.08.003. Epub 2016 Aug 30. Curr Opin Immunol. 2016. PMID: 27588918 Review.
Cited by
-
SARS-CoV-2 vaccination unmasks distinct immune dysfunctions across lymphoma subtypes and therapies.Res Sq [Preprint]. 2025 Jul 4:rs.3.rs-7016519. doi: 10.21203/rs.3.rs-7016519/v1. Res Sq. 2025. PMID: 40630518 Free PMC article. Preprint.
-
Immune aging and infectious diseases.Chin Med J (Engl). 2024 Dec 20;137(24):3010-3049. doi: 10.1097/CM9.0000000000003410. Epub 2024 Dec 16. Chin Med J (Engl). 2024. PMID: 39679477 Free PMC article. Review.
-
Mechanisms underpinning poor antibody responses to vaccines in ageing.Immunol Lett. 2022 Jan;241:1-14. doi: 10.1016/j.imlet.2021.11.001. Epub 2021 Nov 10. Immunol Lett. 2022. PMID: 34767859 Free PMC article. Review.
-
Heterogeneity of memory T cells in aging.Front Immunol. 2023 Aug 18;14:1250916. doi: 10.3389/fimmu.2023.1250916. eCollection 2023. Front Immunol. 2023. PMID: 37662959 Free PMC article. Review.
-
Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies.NPJ Vaccines. 2024 Apr 10;9(1):77. doi: 10.1038/s41541-024-00874-4. NPJ Vaccines. 2024. PMID: 38600250 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

