GIPR antagonist antibodies conjugated to GLP-1 peptide are bispecific molecules that decrease weight in obese mice and monkeys
- PMID: 34095876
- PMCID: PMC8149376
- DOI: 10.1016/j.xcrm.2021.100263
GIPR antagonist antibodies conjugated to GLP-1 peptide are bispecific molecules that decrease weight in obese mice and monkeys
Abstract
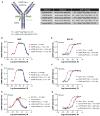
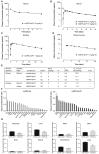
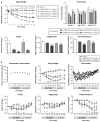
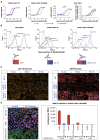
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) regulate glucose and energy homeostasis. Targeting both pathways with GIP receptor (GIPR) antagonist antibody (GIPR-Ab) and GLP-1 receptor (GLP-1R) agonist, by generating GIPR-Ab/GLP-1 bispecific molecules, is an approach for treating obesity and its comorbidities. In mice and monkeys, these molecules reduce body weight (BW) and improve many metabolic parameters. BW loss is greater with GIPR-Ab/GLP-1 than with GIPR-Ab or a control antibody conjugate, suggesting synergistic effects. GIPR-Ab/GLP-1 also reduces the respiratory exchange ratio in DIO mice. Simultaneous receptor binding and rapid receptor internalization by GIPR-Ab/GLP-1 amplify endosomal cAMP production in recombinant cells expressing both receptors. This may explain the efficacy of the bispecific molecules. Overall, our GIPR-Ab/GLP-1 molecules promote BW loss, and they may be used for treating obesity.
Keywords: Cyclic adenosine monophosphate; antibody; cAMP; diet-induced obese mice; glucagon-like peptide-1; glucose-dependent insulinotropic polypeptide; monkeys; obesity; weight loss.
© 2021 Amgen Inc.
Conflict of interest statement
The authors declare no competing interests. All authors are employees and shareholders of Amgen. A patent related to this work has been filed: International Application No. PCT/US18/13918.
Figures



Comment in
-
What combines best with GLP-1 for obesity treatment: GIP receptor agonists or antagonists?Cell Rep Med. 2021 May 18;2(5):100284. doi: 10.1016/j.xcrm.2021.100284. eCollection 2021 May 18. Cell Rep Med. 2021. PMID: 34095885 Free PMC article.
References
-
- Finkelstein E.A., Trogdon J.G., Cohen J.W., Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. (Millwood) 2009;28:w822–w831. - PubMed
-
- Valsamakis G., Konstantakou P., Mastorakos G. New targets for drug treatment of obesity. Annu. Rev. Pharmacol. Toxicol. 2017;57:585–605. - PubMed
-
- Drucker D.J. The biology of incretin hormones. Cell Metab. 2006;3:153–165. - PubMed
-
- Inagaki N., Seino Y., Takeda J., Yano H., Yamada Y., Bell G.I., Eddy R.L., Fukushima Y., Byers M.G., Shows T.B. Gastric inhibitory polypeptide: structure and chromosomal localization of the human gene. Mol. Endocrinol. 1989;3:1014–1021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

