Modifying gut integrity and microbiome in children with severe acute malnutrition using legume-based feeds (MIMBLE): A pilot trial
- PMID: 34095882
- PMCID: PMC8149470
- DOI: 10.1016/j.xcrm.2021.100280
Modifying gut integrity and microbiome in children with severe acute malnutrition using legume-based feeds (MIMBLE): A pilot trial
Abstract
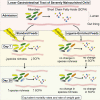
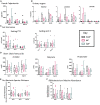
Case fatality among African children with severe acute malnutrition remains high. We report a 3-arm pilot trial in 58 Ugandan children, comparing feeds targeting disordered gastrointestinal function containing cowpea (CpF, n = 20) or inulin (InF, n = 20) with conventional feeds (ConF, n = 18). Baseline measurements of gut permeability (lactulose:mannitol ratio 1.19 ± SD 2.00), inflammation (fecal calprotectin 539.0 μg/g, interquartile range [IQR] 904.8), and satiety (plasma polypeptide YY 62.6 pmol/l, IQR 110.3) confirm gastrointestinal dysfunction. By day 28, no differences are observable in proportion achieving weight gain >5 g/kg/day (87%, 92%, 86%; p > 0.05), mortality (16%, 30%, 17%; p > 0.05), or edema resolution (83%, 54%, 91%; p > 0.05) among CpF, InF, and ConF. Decreased fecal bacterial richness from day 1 (abundance-based coverage estimator [ACE] 53.2) to day 7 (ACE 40.8) is observed only in ConF (p = 0.025). Bifidobacterium relative abundance increases from day 7 (5.8% ± 8.6%) to day 28 (10.9% ± 8.7%) in CpF (corrected p = 1.000). Legume-enriched feeds support aspects of gut function and the microbiome. Trial registration PACTR201805003381361.
Keywords: 16S rRNA; African children; NMR spectroscopy; clinical trial; gut barrier dysfunction; gut hormones; metabolome; microbiome; nutritional feeds; severe malnutrition; short-chain fatty acid.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- World Health Organization . 2017 ed. World Health Organization; 2017. Children: reducing mortality. 01/10.
-
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M., Ezzati M., Grantham-McGregor S., Katz J., Martorell R., Uauy R., Maternal and Child Nutrition Study Group Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. - PubMed
-
- Gachau S., Irimu G., Ayieko P., Akech S., Agweyu A., English M., Clinical Information Network Author Group Prevalence, outcome and quality of care among children hospitalized with severe acute malnutrition in Kenyan hospitals: A multi-site observational study. PLoS ONE. 2018;13:e0197607. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

