PFC mTOR signaling as a biological signature for cognitive deficits in bipolar disorder without psychosis
- PMID: 34095884
- PMCID: PMC8149463
- DOI: 10.1016/j.xcrm.2021.100282
PFC mTOR signaling as a biological signature for cognitive deficits in bipolar disorder without psychosis
Abstract
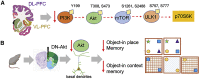
Vanderplow et al.1 report decreased PFC Akt-mTOR signaling in males with bipolar disorder (BD) without psychosis compared with those with psychosis, possibly related to cognitive deficits. Understanding how cognition differs between these BD subtypes clinically and biologically remains a challenge.
© 2021 The Author(s).
Figures
Comment on
-
Akt-mTOR hypoactivity in bipolar disorder gives rise to cognitive impairments associated with altered neuronal structure and function.Neuron. 2021 May 5;109(9):1479-1496.e6. doi: 10.1016/j.neuron.2021.03.008. Epub 2021 Mar 24. Neuron. 2021. PMID: 33765445 Free PMC article.
Similar articles
-
Cognitive deficits in bipolar disorders: Implications for emotion.Clin Psychol Rev. 2018 Feb;59:126-136. doi: 10.1016/j.cpr.2017.11.006. Epub 2017 Nov 21. Clin Psychol Rev. 2018. PMID: 29195773 Free PMC article. Review.
-
Akt-mTOR hypoactivity in bipolar disorder gives rise to cognitive impairments associated with altered neuronal structure and function.Neuron. 2021 May 5;109(9):1479-1496.e6. doi: 10.1016/j.neuron.2021.03.008. Epub 2021 Mar 24. Neuron. 2021. PMID: 33765445 Free PMC article.
-
Differential impairment of social cognition factors in bipolar disorder with and without psychotic features and schizophrenia.J Psychiatr Res. 2013 Dec;47(12):2004-10. doi: 10.1016/j.jpsychires.2013.09.010. Epub 2013 Sep 30. J Psychiatr Res. 2013. PMID: 24112946
-
The impact of psychosis on brain anatomy in bipolar disorder: A structural MRI study.J Affect Disord. 2018 Jun;233:100-109. doi: 10.1016/j.jad.2017.11.092. Epub 2017 Nov 29. J Affect Disord. 2018. PMID: 29223329
-
Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study.J Affect Disord. 2010 Dec;127(1-3):1-9. doi: 10.1016/j.jad.2010.02.117. Epub 2010 Mar 15. J Affect Disord. 2010. PMID: 20231037
Cited by
-
Cellular Metabolism: A Fundamental Component of Degeneration in the Nervous System.Biomolecules. 2023 May 11;13(5):816. doi: 10.3390/biom13050816. Biomolecules. 2023. PMID: 37238686 Free PMC article. Review.
-
The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk.Front Immunol. 2023 Nov 8;14:1273570. doi: 10.3389/fimmu.2023.1273570. eCollection 2023. Front Immunol. 2023. PMID: 38022638 Free PMC article. Review.
-
The Metabolic Basis for Nervous System Dysfunction in Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease.Curr Neurovasc Res. 2023;20(3):314-333. doi: 10.2174/1567202620666230721122957. Curr Neurovasc Res. 2023. PMID: 37488757 Free PMC article.
-
Cognitive Impairment in Multiple Sclerosis.Bioengineering (Basel). 2023 Jul 23;10(7):871. doi: 10.3390/bioengineering10070871. Bioengineering (Basel). 2023. PMID: 37508898 Free PMC article. Review.
References
-
- Vanderplow A.M., Eagle A.L., Kermath B.A., Bjornson K.J., Robison A.J., Cahill M.E. Akt-mTOR hypoactivity in bipolar disorder gives rise to cognitive impairments associated with altered neuronal structure and function. Neuron. 2021;2021 doi: 10.1016/j.neuron.2021.03.008. S0896-6273(21)00156-2. - DOI - PMC - PubMed
-
- Abdallah C.G., Averill L.A., Gueorguieva R., Goktas S., Purohit P., Ranganathan M., Sherif M., Ahn K.-H., D’Souza D.C., Rormica R. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology. 2020;45:990–997. doi: 10.1038/s41386-020-0644-9. - DOI - PMC - PubMed
-
- Abelaira H.M., Réus G.Z., Ignácio Z.M., Dos Santos M.A., de Moura A.B., Matos D., Demo J.P., da Silva J.B., Michels M., Abatti M. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J. Psychiatr. Res. 2017;87:81–87. doi: 10.1016/j.jpsychires.2016.12.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

