Neuraminidase and SIGLEC15 modulate the host defense against pulmonary aspergillosis
- PMID: 34095887
- PMCID: PMC8149467
- DOI: 10.1016/j.xcrm.2021.100289
Neuraminidase and SIGLEC15 modulate the host defense against pulmonary aspergillosis
Abstract
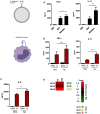
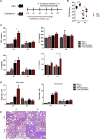
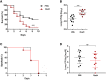
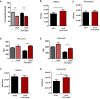
Influenza-associated pulmonary aspergillosis (IAPA) has been reported increasingly since the advent of use of neuraminidase (NA) inhibitors following the 2009 influenza pandemic. We hypothesize that blocking host NA modulates the immune response against Aspergillus fumigatus. We demonstrate that NA influences the host response against A. fumigatus in vitro and that oseltamivir increases the susceptibility of mice to pulmonary aspergillosis. Oseltamivir impairs the mouse splenocyte and human peripheral blood mononuclear cell (PBMC) killing capacity of A. fumigatus, and adding NA restores this defect in PBMCs. Furthermore, the sialic acid-binding receptor SIGLEC15 is upregulated in PBMCs stimulated with A. fumigatus. Silencing of SIGLEC15 decrease PBMC killing of A. fumigatus. We provide evidence that host NA activity and sialic acid recognition are important for anti-Aspergillus defense. NA inhibitors might predispose individuals with severe influenza to invasive aspergillosis. These data shed light on the pathogenesis of invasive fungal infections and may identify potential therapeutic targets.
Keywords: SIGLEC15; aspergillosis; neuraminidase; oseltamivir.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Early oseltamivir reduces risk for influenza-associated aspergillosis in a double-hit murine model.Virulence. 2021 Dec;12(1):2493-2508. doi: 10.1080/21505594.2021.1974327. Virulence. 2021. PMID: 34546839 Free PMC article.
-
Immunotherapy with nebulized pattern recognition receptor agonists restores severe immune paralysis and improves outcomes in mice with influenza-associated pulmonary aspergillosis.mBio. 2025 May 14;16(5):e0406124. doi: 10.1128/mbio.04061-24. Epub 2025 Apr 8. mBio. 2025. PMID: 40197039 Free PMC article.
-
Development of a Corticosteroid-Immunosuppressed Mouse Model to Study the Pathogenesis and Therapy of Influenza-Associated Pulmonary Aspergillosis.J Infect Dis. 2023 Apr 12;227(7):901-906. doi: 10.1093/infdis/jiad001. J Infect Dis. 2023. PMID: 36611269 Free PMC article.
-
Lung and gut microbiomes in pulmonary aspergillosis: Exploring adjunctive therapies to combat the disease.Front Immunol. 2022 Aug 12;13:988708. doi: 10.3389/fimmu.2022.988708. eCollection 2022. Front Immunol. 2022. PMID: 36032147 Free PMC article. Review.
-
Neuraminidase inhibitors as antiviral agents.Curr Drug Targets Infect Disord. 2005 Dec;5(4):401-9. doi: 10.2174/156800505774912884. Curr Drug Targets Infect Disord. 2005. PMID: 16535861 Review.
Cited by
-
Siglec15 in blood system diseases: from bench to bedside.Front Immunol. 2024 Dec 4;15:1490505. doi: 10.3389/fimmu.2024.1490505. eCollection 2024. Front Immunol. 2024. PMID: 39697338 Free PMC article. Review.
-
Dysregulated Pulmonary Inflammatory Responses Exacerbate the Outcome of Secondary Aspergillosis Following Influenza.bioRxiv [Preprint]. 2023 Jun 30:2023.06.27.546808. doi: 10.1101/2023.06.27.546808. bioRxiv. 2023. Update in: mBio. 2023 Oct 31;14(5):e0163323. doi: 10.1128/mbio.01633-23. PMID: 37425745 Free PMC article. Updated. Preprint.
-
Defective antifungal immunity in patients with COVID-19.Front Immunol. 2022 Nov 30;13:1080822. doi: 10.3389/fimmu.2022.1080822. eCollection 2022. Front Immunol. 2022. PMID: 36531987 Free PMC article. Review.
-
Integrating genetic and immune factors to uncover pathogenetic mechanisms of viral-associated pulmonary aspergillosis.mBio. 2024 Jun 12;15(6):e0198223. doi: 10.1128/mbio.01982-23. Epub 2024 Apr 23. mBio. 2024. PMID: 38651925 Free PMC article. Review.
-
Incidence, risk factors and mortality of invasive pulmonary aspergillosis in patients with influenza: A systematic review and meta-analysis.Mycoses. 2022 Feb;65(2):152-163. doi: 10.1111/myc.13410. Epub 2021 Dec 22. Mycoses. 2022. PMID: 34882852 Free PMC article.
References
-
- van de Veerdonk F.L., Kolwijck E., Lestrade P.P., Hodiamont C.J., Rijnders B.J., van Paassen J., Haas P.J., Oliveira Dos Santos C., Kampinga G.A., Bergmans D.C., Dutch Mycoses Study Group Influenza-Associated Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2017;196:524–527. - PubMed
-
- Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., Lagrou K., Verweij P.E., Van de Veerdonk F.L., Gommers D., Dutch-Belgian Mycosis study group Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 2018;6:782–792. - PubMed
-
- Miyagi T., Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–896. - PubMed
-
- Amith S.R., Jayanth P., Franchuk S., Siddiqui S., Seyrantepe V., Gee K., Basta S., Beyaert R., Pshezhetsky A.V., Szewczuk M.R. Dependence of pathogen molecule-induced Toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj. J. 2009;26:1197–1212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

