Non-conventional Behavior of a 2,1-Benzazaphosphole: Heterodiene or Hidden Phosphinidene?
- PMID: 34096106
- PMCID: PMC8518707
- DOI: 10.1002/chem.202101686
Non-conventional Behavior of a 2,1-Benzazaphosphole: Heterodiene or Hidden Phosphinidene?
Abstract
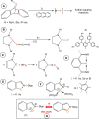
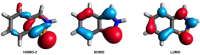
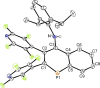
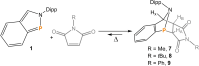
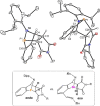
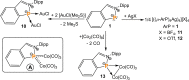
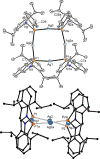
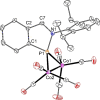
The titled 2,1-benzazaphosphole (1) (i. e. ArP, where Ar=2-(DippN=CH)C6 H4 , Dipp=2,6-iPr2 C6 H3 ) showed a spectacular reactivity behaving both as a reactive heterodiene in hetero-Diels-Alder (DA) reactions or as a hidden phosphinidene in the coordination toward selected transition metals (TMs). Thus, 1 reacts with electron-deficient alkynes RC≡CR (R=CO2 Me, C5 F4 N) giving 1-phospha-1,4-dihydro-iminonaphthalenes 2 and 3, that undergo hydrogen migration producing 1-phosphanaphthalenes 4 and 5. Compound 1 is also able to activate the C=C double bond in selected N-alkyl/aryl-maleimides RN(C(O)CH)2 (R=Me, tBu, Ph) resulting in the addition products 7-9 with bridged bicyclic [2.2.1] structures. The binding of the maleimides to 1 is semi-reversible upon heating. By contrast, when 1 was treated with selected TM complexes, it serves as a 4e donor bridging two TMs thus producing complexes [μ-ArP(AuCl)2 ] (10), [(μ-ArP)4 Ag4 ][X]4 (X=BF4 (11), OTf (12)) and [μ-ArP(Co2 (CO)6 )] (13). The structure and electron distribution of the starting material 1 as well as of other compounds were also studied from the theoretical point of view.
Keywords: Diels-Alder reactions; NRT study; heterodienes; phospha-heterocycles; phosphinidenes.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
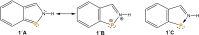




Similar articles
-
Hetero Diels-Alder Reactions of Masked Dienes Containing Heavy Group 15 Elements.Chemistry. 2020 Jan 22;26(5):1144-1154. doi: 10.1002/chem.201904953. Epub 2020 Jan 17. Chemistry. 2020. PMID: 31769071
-
Reversible C=C Bond Activation by an Intramolecularly Coordinated Antimony(I) Compound.Chemistry. 2019 Oct 8;25(56):12884-12888. doi: 10.1002/chem.201902968. Epub 2019 Aug 28. Chemistry. 2019. PMID: 31353625
-
From a 2,1-Benzazaarsole to Elusive 1-Arsanaphthalenes in One Step.Chemistry. 2019 Apr 17;25(22):5668-5671. doi: 10.1002/chem.201900805. Epub 2019 Mar 27. Chemistry. 2019. PMID: 30861223
-
Homometallic rare-Earth metal phosphinidene clusters: synthesis and reactivity.Angew Chem Int Ed Engl. 2014 Jan 20;53(4):1053-6. doi: 10.1002/anie.201307422. Epub 2013 Dec 5. Angew Chem Int Ed Engl. 2014. PMID: 24311456
-
Neutral and zwitterionic low-coordinate titanium complexes bearing the terminal phosphinidene functionality. Structural, spectroscopic, theoretical, and catalytic studies addressing the Ti-P multiple bond.J Am Chem Soc. 2006 Oct 18;128(41):13575-85. doi: 10.1021/ja064853o. J Am Chem Soc. 2006. PMID: 17031972
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

