Mechanistic Insight into How PEGylation Reduces the Efficacy of pH-Sensitive Liposomes from Molecular Dynamics Simulations
- PMID: 34096310
- PMCID: PMC8289284
- DOI: 10.1021/acs.molpharmaceut.1c00122
Mechanistic Insight into How PEGylation Reduces the Efficacy of pH-Sensitive Liposomes from Molecular Dynamics Simulations
Abstract
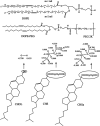
Liposome-based drug delivery systems composed of DOPE stabilized with cholesteryl hemisuccinate (CHMS) have been proposed as a drug delivery mechanism with pH-triggered release as the anionic form (CHSa) is protonated (CHS) at reduced pH; PEGylation is known to decrease this pH sensitivity. In this manuscript, we set out to use molecular dynamics (MD) simulations with a model with all-atom resolution to provide insight into why incorporation of poly(ethyleneglycol) (PEG) into DOPE-CHMS liposomes reduces their pH sensitivity; we also address two additional questions: (1) How CHSa stabilizes DOPE bilayers into a lamellar conformation at a physiological pH of 7.4? and (2) how the change from CHSa to CHS at acidic pH triggers the destabilization of DOPE bilayers? We found that (A) CHSa stabilizes the DOPE lipid membrane by increasing the hydrophilicity of the bilayer surface, (B) when CHSa changes to CHS by pH reduction, DOPE bilayers are destabilized due to a reduction in bilayer hydrophilicity and a reduction in the area per lipid, and (C) PEG stabilizes DOPE bilayers into the lamellar phase, thus reducing the pH sensitivity of the liposomes by increasing the area per lipid through penetration into the bilayer, which is our main focus.
Keywords: PEGylated pH-sensitive liposomes; bilayer hydrophilicity; cholesteryl hemisuccinate; molecular dynamics simulations; phase transition.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Lipid nature and their influence on opening of redox-active liposomes.Langmuir. 2013 Jun 4;29(22):6615-23. doi: 10.1021/la304340e. Epub 2013 May 22. Langmuir. 2013. PMID: 23698020 Free PMC article.
-
Fusogenic activity of PEGylated pH-sensitive liposomes.J Liposome Res. 2012 Jun;22(2):148-57. doi: 10.3109/08982104.2011.633267. Epub 2011 Dec 10. J Liposome Res. 2012. PMID: 22149717
-
Ursolic acid incorporation does not prevent the formation of a non-lamellar phase in pH-sensitive and long-circulating liposomes.Langmuir. 2014 Dec 23;30(50):15083-90. doi: 10.1021/la502977j. Epub 2014 Dec 9. Langmuir. 2014. PMID: 25490253
-
On the formulation of pH-sensitive liposomes with long circulation times.Adv Drug Deliv Rev. 2004 Apr 23;56(7):947-65. doi: 10.1016/j.addr.2003.10.038. Adv Drug Deliv Rev. 2004. PMID: 15066754 Review.
-
Rational design of liposomal drug delivery systems, a review: Combined experimental and computational studies of lipid membranes, liposomes and their PEGylation.Biochim Biophys Acta. 2016 Oct;1858(10):2334-2352. doi: 10.1016/j.bbamem.2016.02.025. Epub 2016 Feb 23. Biochim Biophys Acta. 2016. PMID: 26915693 Review.
Cited by
-
Establishment and evaluation of glucose-modified nanocomposite liposomes for the treatment of cerebral malaria.J Nanobiotechnology. 2022 Jul 6;20(1):318. doi: 10.1186/s12951-022-01493-8. J Nanobiotechnology. 2022. PMID: 35794597 Free PMC article.
-
When conventional approach in toxicity assays falls short for nanomedicines: a case study with nanoemulsions.Drug Deliv Transl Res. 2025 Aug;15(8):2814-2832. doi: 10.1007/s13346-024-01776-7. Epub 2025 Jan 8. Drug Deliv Transl Res. 2025. PMID: 39779651 Free PMC article.
-
Nanotechnology-Driven Drug Delivery Systems for Lung Cancer: Computational Advances and Clinical Perspectives.Thorac Cancer. 2025 Jul;16(14):e70134. doi: 10.1111/1759-7714.70134. Thorac Cancer. 2025. PMID: 40682256 Free PMC article. Review.
-
αvβ3 Integrin and Folate-Targeted pH-Sensitive Liposomes with Dual Ligand Modification for Metastatic Breast Cancer Treatment.Bioengineering (Basel). 2024 Aug 7;11(8):800. doi: 10.3390/bioengineering11080800. Bioengineering (Basel). 2024. PMID: 39199757 Free PMC article.
-
Computational Methods for Modeling Lipid-Mediated Active Pharmaceutical Ingredient Delivery.Mol Pharm. 2025 Mar 3;22(3):1110-1141. doi: 10.1021/acs.molpharmaceut.4c00744. Epub 2025 Jan 29. Mol Pharm. 2025. PMID: 39879096 Free PMC article. Review.
References
-
- MacLachlan I.Liposomal Formulations For Nucleic Acid Delivery. Antisense Drug Technology: Principles, Strategies, Applications; Crooke S. T., Ed.; CRC Press, 2007; pp 237–270.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

