Bioprinting of kidney in vitro models: cells, biomaterials, and manufacturing techniques
- PMID: 34096573
- PMCID: PMC8365327
- DOI: 10.1042/EBC20200158
Bioprinting of kidney in vitro models: cells, biomaterials, and manufacturing techniques
Abstract
The number of patients with end-stage renal disease is continuously increasing worldwide. The only therapies for these patients are dialysis and organ transplantation, but the latter is limited due to the insufficient number of donor kidneys available. Research in kidney disease and alternative therapies are therefore of outmost importance. In vitro models that mimic human kidney functions are essential to provide better insights in disease and ultimately novel therapies. Bioprinting techniques have been increasingly used to create models with some degree of function, but their true potential is yet to be achieved. Bioprinted renal tissues and kidney-like constructs presents challenges, for example, choosing suitable renal cells and biomaterials for the formulation of bioinks. In addition, the fabrication of complex renal biological structures is still a major bottleneck. Advances in pluripotent stem cell-derived renal progenitors has contributed to in vivo-like rudiment structures with multiple renal cells, and these started to make a great impact on the achieved models. Natural- or synthetic-based biomaterial inks, such as kidney-derived extracellular matrix and gelatin-fibrin hydrogels, which show the potential to partially replicate in vivo-like microenvironments, have been largely investigated for bioprinting. As the field progresses, technological, biological and biomaterial developments will be required to yield fully functional in vitro tissues that can contribute to a better understanding of renal disease, to improve predictability in vitro of novel therapeutics, and to facilitate the development of alternative regenerative or replacement treatments. In this review, we resume the main advances on kidney in vitro models reported so far.
Keywords: Bioprinting; biomaterials; in vitro models; kidney.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
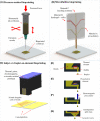




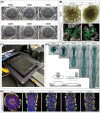

References
-
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M.et al. . (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am. Ed. 395, 709–733 10.1016/S0140-6736(20)30045-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

