Human prefoldin modulates co-transcriptional pre-mRNA splicing
- PMID: 34096575
- PMCID: PMC8216451
- DOI: 10.1093/nar/gkab446
Human prefoldin modulates co-transcriptional pre-mRNA splicing
Abstract
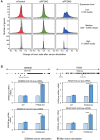
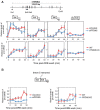
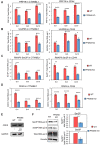
Prefoldin is a heterohexameric complex conserved from archaea to humans that plays a cochaperone role during the co-translational folding of actin and tubulin monomers. Additional functions of prefoldin have been described, including a positive contribution to transcription elongation and chromatin dynamics in yeast. Here we show that prefoldin perturbations provoked transcriptional alterations across the human genome. Severe pre-mRNA splicing defects were also detected, particularly after serum stimulation. We found impairment of co-transcriptional splicing during transcription elongation, which explains why the induction of long genes with a high number of introns was affected the most. We detected genome-wide prefoldin binding to transcribed genes and found that it correlated with the negative impact of prefoldin depletion on gene expression. Lack of prefoldin caused global decrease in Ser2 and Ser5 phosphorylation of the RNA polymerase II carboxy-terminal domain. It also reduced the recruitment of the CTD kinase CDK9 to transcribed genes, and the association of splicing factors PRP19 and U2AF65 to chromatin, which is known to depend on CTD phosphorylation. Altogether the reported results indicate that human prefoldin is able to act locally on the genome to modulate gene expression by influencing phosphorylation of elongating RNA polymerase II, and thereby regulating co-transcriptional splicing.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Arranz R., Martín-Benito J., Valpuesta J.M.. Structure and function of the cochaperone Prefoldin. Prefoldins: The New Chaperones. 2018; 119–131. - PubMed
-
- Chávez S., Puerto-Camacho P.. Prefoldins. eLS. 2016; Chichester, UK: John Wiley & Sons Ltd; 1–8.
-
- Lundin V.F., Leroux M.R., Stirling P.C.. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010; 35:288–297. - PubMed
-
- Boulon S., Bertrand E., Pradet-Balade B.. HSP90 and the R2TP co-chaperone complex: Building multi-protein machineries essential for cell growth and gene expression. RNA Biol. 2012; 9:148–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

