Orphan nuclear receptor 4A1 (NR4A1) and novel ligands
- PMID: 34096590
- PMCID: PMC11410023
- DOI: 10.1042/EBC20200164
Orphan nuclear receptor 4A1 (NR4A1) and novel ligands
Abstract
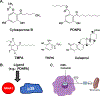
The nuclear receptor (NR) superfamily of transcription factors encodes expression of 48 human genes that are important for maintaining cellular homeostasis and in pathophysiology, and this has been observed for all sub-families including orphan receptors for which endogenous ligands have not yet been identified. The orphan NR4A1 (Nur77 and TR3) and other members of this sub-family (NR4A2 and NR4A3) are immediate early genes induced by diverse stressors, and these receptors play an important role in the immune function and are up-regulated in some inflammatory diseases including solid tumors. Although endogenous ligands for NR4A have not been identified, several different classes of compounds have been characterized as NR4A1 ligands that bind the receptor. These compounds include cytosporone B and structurally related analogs, bis-indole derived (CDIM) compounds, the triterpenoid celastrol and a number of other chemicals including polyunsaturated fatty acids. NR4A1 ligands bind different regions/surfaces of NR4A1 and exhibit selective NR4A1 modulator (SNR4AM) activities that are dependent on ligand structure and cell/tissue context. NR4A1 ligands exhibit pharmacologic activities in studies on cancer, endometriosis metabolic and inflammatory diseases and are promising agents with clinical potential for treating multiple diseases.
Keywords: Agonists; Antagonists; Ligands; NR4A1.
© 2021 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Figures
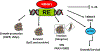
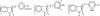

Similar articles
-
Natural products and synthetic analogs as selective orphan nuclear receptor 4A (NR4A) modulators.Histol Histopathol. 2024 May;39(5):543-556. doi: 10.14670/HH-18-689. Epub 2023 Dec 13. Histol Histopathol. 2024. PMID: 38116863 Free PMC article. Review.
-
The Paradoxical Roles of Orphan Nuclear Receptor 4A (NR4A) in Cancer.Mol Cancer Res. 2021 Feb;19(2):180-191. doi: 10.1158/1541-7786.MCR-20-0707. Epub 2020 Oct 26. Mol Cancer Res. 2021. PMID: 33106376 Free PMC article. Review.
-
Nuclear receptor 4A (NR4A) family - orphans no more.J Steroid Biochem Mol Biol. 2016 Mar;157:48-60. doi: 10.1016/j.jsbmb.2015.04.016. Epub 2015 Apr 23. J Steroid Biochem Mol Biol. 2016. PMID: 25917081 Free PMC article.
-
NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology.Arterioscler Thromb Vasc Biol. 2010 Aug;30(8):1535-41. doi: 10.1161/ATVBAHA.109.191163. Arterioscler Thromb Vasc Biol. 2010. PMID: 20631354 Free PMC article. Review.
-
Dual nuclear receptor 4A1 (NR4A1/NR4A2) ligands inhibit glioblastoma growth and target TWIST1.Mol Pharmacol. 2025 Feb;107(2):100009. doi: 10.1016/j.molpha.2024.100009. Epub 2024 Dec 31. Mol Pharmacol. 2025. PMID: 40023516 Free PMC article.
Cited by
-
Towards a unified molecular mechanism for liganddependent activation of NR4A-RXR heterodimers.bioRxiv [Preprint]. 2025 Mar 19:2025.03.19.642122. doi: 10.1101/2025.03.19.642122. bioRxiv. 2025. PMID: 40166180 Free PMC article. Preprint.
-
NR4A Family Genes: A Review of Comprehensive Prognostic and Gene Expression Profile Analysis in Breast Cancer.Front Oncol. 2022 Apr 25;12:777824. doi: 10.3389/fonc.2022.777824. eCollection 2022. Front Oncol. 2022. PMID: 35547878 Free PMC article.
-
Inhibition of Nur77 expression and translocation by compound B6 reduces ER stress and alleviates cigarette smoke-induced inflammation and injury in bronchial epithelial cells.Front Pharmacol. 2023 Jun 19;14:1200110. doi: 10.3389/fphar.2023.1200110. eCollection 2023. Front Pharmacol. 2023. PMID: 37405051 Free PMC article.
-
Nuclear receptors: from molecular mechanisms to therapeutics.Essays Biochem. 2021 Dec 17;65(6):847-856. doi: 10.1042/EBC20210020. Essays Biochem. 2021. PMID: 34825698 Free PMC article.
-
Nuclear receptors as novel regulators that modulate cancer radiosensitivity and normal tissue radiotoxicity.Mol Cancer. 2025 May 30;24(1):155. doi: 10.1186/s12943-025-02362-2. Mol Cancer. 2025. PMID: 40442680 Free PMC article. Review.
References
-
- Gallastegui N, Mackinnon JA, Fletterick RJ, Estebanez-Perpina E. Advances in our structural understanding of orphan nuclear receptors. Trends Biochem Sci. 2015;40(1):25–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

