Clinical Outcomes and Racial Disparities in Metastatic Hormone-Sensitive Prostate Cancer in the Era of Novel Treatment Options
- PMID: 34096667
- PMCID: PMC8571757
- DOI: 10.1002/onco.13848
Clinical Outcomes and Racial Disparities in Metastatic Hormone-Sensitive Prostate Cancer in the Era of Novel Treatment Options
Abstract
Background: Docetaxel (DOC) and abiraterone (ABI) in the upfront setting have separately improved clinical outcomes for metastatic hormone-sensitive prostate cancer (mHSPC), but there are no studies comparing drug efficacies or the influence of racial disparities.
Materials and methods: We performed a retrospective multicenter review from Winship Cancer Institute at Emory University and Georgia Cancer Center for Excellence at Grady Memorial Hospital (2014-2020) for patients with mHSPC treated with either upfront DOC or ABI. Outcomes evaluated were overall survival (OS), progression-free survival (PFS), and prostate-specific antigen complete response (PSA CR).
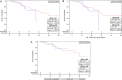
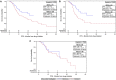
Results: A total of 168 patients were included, consisting of 92 (54.8%) Black patients and 76 (45.2%) non-Black patients (69 White and 7 Asian or Hispanic). Ninety-four (56%) received DOC and 74 (44%) received ABI. Median follow-up time was 22.8 months with data last reviewed June 2020. For OS, there was no significant difference between ABI versus DOC and Black versus non-Black patients. For PFS, DOC was associated with hazard ratio (HR) 1.7 compared with ABI for all patients based on univariate association and HR 2.27 compared with ABI for Black patients on multivariable analysis. For PSA CR, Black patients were less likely to have a CR (odds ratio [OR] = 0.27).
Conclusion: ABI and DOC have similar OS with a trend toward better PFS for ABI in a cohort composed of 54% Black patients. Racial disparities were observed as prolonged PFS for Black patients treated with ABI, more so compared with all patients, and less PSA CR for Black patients. A prospective trial comparing available upfront therapies in a diverse racial population is needed to help guide clinical decision-making in the era of novel treatment options.
Implications for practice: Overall survival is similar for abiraterone and docetaxel when used as upfront therapy in metastatic hormone-sensitive prostate cancer in a cohort composed of 54% Black patients. There is a trend towards improved progression-free survival for abiraterone in all patients and Black patients. Non-Black patients were more likely to achieve prostate-specific antigen (PSA) complete response regardless of upfront therapy.
Keywords: Abiraterone; Castration-sensitive prostate cancer; Docetaxel; Racial disparities; Upfront therapy.
© 2021 AlphaMed Press.
Conflict of interest statement
Figures
References
-
- National Cancer Institute . Cancer Stat Facts: Common Cancer Sites. Available at https://seer.cancer.gov/statfacts/html/common.html. Accessed July 6, 2020.
-
- Dalela D, Sun M, Diaz M et al. Contemporary trends in the incidence of metastatic prostate cancer among US men: Results from nationwide analyses. Eur Urol Focus 2019;5:77–90. - PubMed
-
- Weiner AB, Matulewicz RS, Eggener SE et al. Increasing incidence of metastatic prostate cancer in the United States (2004‐2013). Prostate Cancer Prostatic Dis 2016;19:395–397. - PubMed
-
- Hahn AW, Higano CS, Taplin M‐E et al. Metastatic castration sensitive prostate cancer: Optimizing patient selection and treatment. Am Soc Clin Oncol Educ Book 2018;38:363–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

