Copper-Catalyzed Three-Component Alkene Carbofunctionalization: C-N, C-O, and C-C Bond Formation from a Single Reaction Platform
- PMID: 34096733
- PMCID: PMC9807022
- DOI: 10.1021/acs.orglett.1c01180
Copper-Catalyzed Three-Component Alkene Carbofunctionalization: C-N, C-O, and C-C Bond Formation from a Single Reaction Platform
Abstract
A general system achieving three-component intermolecular carbofunctionalization of alkenes is presented, including carboetherification, carboesterification, carboarylation, and carboamination. The scope of the reaction is presented with respect to the carbon electrophile, the olefin, and the nucleophile. Furthermore, the synthesis of γ-lactams via a carboamination reaction is demonstrated in a telescoped three-step protocol.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

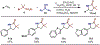
References
-
-
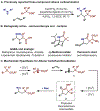
Selected one-component alkene carboamination examples:
- Wdowik T; Galster SL; Carmo RLL; Chemler SR Enantioselective, Aerobic Copper-Catalyzed Intramolecular Carboamination and Carboetherification of Unactivated Alkenes. ACS Catal. 2020, 10, 8535–8541. - PMC - PubMed
- Miao L; Haque I; Manzoni MR; Tham WS; Chemler SR Diastereo- and Enantioselective Copper-Catalyzed Intramolecular Carboamination of Alkenes for the Synthesis of Hexahydro-1H-benz[f]indoles. Org. Lett 2010, 12, 4739–4741. - PMC - PubMed
- He W; Yip K-T; Zhu N-Y; Yang D Pd(II)/tBu-quinolineoxazoline: An Air-Stable and Modular Chiral Catalyst System for Enantioselective Oxidative Cascade Cyclization. Org. Lett 2009, 11, 5626–5628. - PubMed
- Zeng W; Chemler SR Copper(II)-Catalyzed Enantioselective Intramolecular Carboamination of Alkenes. J. Am. Chem. Soc 2007, 129, 12948–12949. - PMC - PubMed
-
-
-
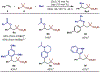
Selected two-component carboamination examples:
- Ozols K; Onodera S; Wozniak L; Cramer N Cobalt(III)-Catalyzed Enantioselective Intermolecular Carboamination by C–H Functionalization. Angew. Chem. Int. Ed 2021, 60, 655–659. - PubMed
- He J; Xue Y; Han B; Zhang C; Wang Y; Zhu S Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem. Int. Ed 2020, 59, 2328–2332. - PubMed
- Um C; Chemler SR Synthesis of 2-Aryl- and 2-Vinylpyrrolidines via Copper-Catalyzed Coupling of Styrenes and Dienes with Potassium β-Aminoethyl Trifluoroborates. Org. Lett 2016, 18, 2515–2518. - PMC - PubMed
- Piou T; Rovis T Rhodium-Catalyzed syn-Carboamination of Alkenes via a Transient Directing Group. Nature, 2015, 527, 86–90. - PMC - PubMed
- Hopkins BA; Wolfe JP Synthesis of Enantiomerically Enriched Imidazolidin-2-ones through Asymmetric Palladium-Catalyzed Alkene Carboamination. Angew. Chem. Int. Ed 2012, 51, 9886–9890. - PMC - PubMed
-
-
-
Selected one-component carboetherification examples:
- Karyakarte SD; Um C; Berhane IA; Chemler SR Synthesis of Spirocyclic Ethers by Enantioselective Copper-Catalyzed Carboetherification of Alkenols. Angew. Chem. Int. Ed 2018, 57, 12921–12924. - PMC - PubMed
- Miller Y; Miao L; Hosseini AS; Chemler SR Copper-Catalyzed Intramolecular Alkene Carboetherification: Synthesis of Fused-Ring and Bridged-Ring Tetrahydrofurans. J. Am. Chem. Soc 2012, 134, 12149–12156. - PMC - PubMed
- Nakhla JS; Kampf JW; Wolfe JP Intramolecular Pd-Catalyzed Carboetherification and Carboamination. Influence of Catalyst Structure on Reaction Mechanism and Product Stereochemistry. J. Am. Chem. Soc 2006, 128, 2893–2901. - PMC - PubMed
-
-
-
Selected two-component carboetherification examples:
- Tsui E; Metrano AJ; Tsuchiya Y; Knowles RR Catalytic Hydroetherification of Unactivated Alkenes Enabled by Proton-Coupled Electron Transfer. Angew. Chem. Int. Ed 2020, 59, 11845–11849. - PMC - PubMed
- Silva AR; Polo EC; Martins NC; Correia CRD Enantioselective Oxy-Heck-Matsuda Arylations: Expeditious Synthesis of Dihydrobenzofuran Systems and Total Synthesis of the Neolignan (−)-Conocarpan. Adv. Synth. Catal 2018, 360, 346–365.
- Chen D; Chemler SR Synthesis of Phthalans via Copper-Catalyzed Enantioselective Cyclization/Carboetherification of 2-Vinylbenzyl Alcohols. Org. Lett 2018, 20, 6453–6456. - PMC - PubMed
- Wolfe JP; Rossi MA Stereoselective Synthesis of Tetrahydrofurans via the Palladium-Catalyzed Reaction of Aryl Bromides with γ-Hydroxy Alkenes: Evidence for an Unusual Intramolecular Olefin Insertion into a Pd(Ar)(OR) Intermediate. J. Am. Chem. Soc 2004, 126, 1620–1621. - PubMed
-
-
-
Selected examples:
- Li Z; Zhang M; Zhang Y; Liu S; Zhao J; Zhang Q Multicomponent Cyclopropane Synthesis Enabled by Cu-Catalyzed Cyclopropene Carbometalation with Organoboron Reagent: Enantioselective Modular Access to Polysubstituted 2-Arylcyclopropylamines. Org. Lett 2019, 21, 5432–5437. - PubMed
- Puyl V; Derosa J; Engle KM Directed, Nickel-Catalyzed Umpolung 1,2-Carboamination of Alkenyl Carbonyl Compounds. ACS Catal. 2019, 9, 224–229.
- Liu Z; Wang Y; Wang Z; Zeng T; Liu P, Engle KM Catalytic Intermolecular Carboamination of Unactivated Alkenes via Directed Aminopalladation. J. Am. Chem. Soc 2017, 139, 11261–11270. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

