Early and late morbidity of local excision after chemoradiotherapy for rectal cancer
- PMID: 34097005
- PMCID: PMC8183183
- DOI: 10.1093/bjsopen/zrab043
Early and late morbidity of local excision after chemoradiotherapy for rectal cancer
Abstract
Background: Local excision (LE) after chemoradiotherapy is a new option in low rectal cancer, but morbidity has never been compared prospectively with total mesorectal excision (TME). Early and late morbidity were compared in patients treated either by LE or TME after neoadjuvant chemoradiotherapy for rectal cancer.
Method: This was a post-hoc analysis from a randomized trial. Patients with clinical T2/T3 low rectal cancer with good response to the chemoradiotherapy and having either LE, LE with eventual completion TME, or TME were considered. Early (1 month) and late (2 years) morbidities were compared between the three groups.
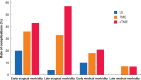
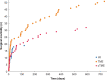
Results: There were no deaths following surgery in any of the three groups. Early surgical morbidity (20 per cent LE versus 36 per cent TME versus 43 per cent completion TME, P = 0.025) and late surgical morbidity (4 per cent versus 33 per cent versus 57 per cent, P < 0.001) were significantly lower in the LE group than in the TME or the completion TME group. of LE, was associated with the lowest rate of early (10 versus 18 versus 21 per cent, P = 0.217) and late medical morbidities (0 versus 7 versus 7 per cent, P = 0.154), although this did not represent a significant difference between the groups. The severity of overall morbidity was significantly lower at 2 years after LE compared with TME or completion TME (4 versus 28 versus 43 per cent grade 3-5, P < 0.001).
Conclusion: The rate of surgical complications after neoadjuvant chemoradiotherapy in the LE group was half that of TME group at 1 month and 10 times lower at 2 years. LE is a safe approach for organ preservation and should be considered as an alternative to watch-and-wait in complete clinical responders and to TME in subcomplete responders.
Trial registration: ClinicalTrials.gov NCT00427375.
© The Author(s) 2021. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures
References
-
- Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 2012;99:1211–1218 - PubMed
-
- Pucciarelli S, De Paoli A, Guerrieri M, La Torre G, Maretto I, De Marchi F et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum 2013;56:1349–1356 - PubMed
-
- Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537–1546 - PMC - PubMed
-
- Smart CJ, Korsgen S, Hill J, Speake D, Levy B, Steward M et al. Multicentre study of short-course radiotherapy and transanal endoscopic microsurgery for early rectal cancer. Br J Surg 2016;103:1069–1075 - PubMed
-
- Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E et al. ; for the CARTS Study Group. Long-term oncologic and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer, The CARTS study. JAMA Surg 2019;154:47–54 - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

