HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research
- PMID: 34097448
- PMCID: PMC8189312
- DOI: 10.1161/CIRCULATIONAHA.120.044221
HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research
Abstract
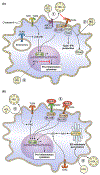
Low high-density lipoprotein cholesterol (HDL-C) characterizes an atherogenic dyslipidemia that reflects adverse lifestyle choices, impaired metabolism, and increased cardiovascular risk. Low HDL-C is also associated with increased risk of inflammatory disorders, malignancy, diabetes, and other diseases. This epidemiologic evidence has not translated to raising HDL-C as a viable therapeutic target, partly because HDL-C does not reflect high-density lipoprotein (HDL) function. Mendelian randomization analyses that have found no evidence of a causal relationship between HDL-C levels and cardiovascular risk have decreased interest in increasing HDL-C levels as a therapeutic target. HDLs comprise distinct subpopulations of particles of varying size, charge, and composition that have several dynamic and context-dependent functions, especially with respect to acute and chronic inflammatory states. These functions include reverse cholesterol transport, inhibition of inflammation and oxidation, and antidiabetic properties. HDLs can be anti-inflammatory (which may protect against atherosclerosis and diabetes) and proinflammatory (which may help clear pathogens in sepsis). The molecular regulation of HDLs is complex, as evidenced by their association with multiple proteins, as well as bioactive lipids and noncoding RNAs. Clinical investigations of HDL biomarkers (HDL-C, HDL particle number, and apolipoprotein A through I) have revealed nonlinear relationships with cardiovascular outcomes, differential relationships by sex and ethnicity, and differential patterns with coronary versus noncoronary events. Novel HDL markers may also have relevance for heart failure, cancer, and diabetes. HDL function markers (namely, cholesterol efflux capacity) are associated with coronary disease, but they remain research tools. Therapeutics that manipulate aspects of HDL metabolism remain the holy grail. None has proven to be successful, but most have targeted HDL-C, not metrics of HDL function. Future therapeutic strategies should focus on optimizing HDL function in the right patients at the optimal time in their disease course. We provide a framework to help the research and clinical communities, as well as funding agencies and stakeholders, obtain insights into current thinking on these topics, and what we predict will be an exciting future for research and development on HDLs.
Keywords: atherosclerosis; biomarkers; inflammation; lipoproteins; lipoproteins, HDL.
Figures

References
-
- Madsen CM, Varbo A and Nordestgaard BG. Novel Insights From Human Studies on the Role of High-Density Lipoprotein in Mortality and Noncardiovascular Disease. Arterioscler Thromb Vasc Biol. 2021;41:128–140. - PubMed
-
- Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A, Jerome WG, Rye KA and Davidson WS. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12176–81. - PMC - PubMed
-
- Wu Z, Gogonea V, Lee X, May RP, Pipich V, Wagner MA, Undurti A, Tallant TC, Baleanu-Gogonea C, Charlton F, Ioffe A, DiDonato JA, Rye KA and Hazen SL. The low resolution structure of ApoA1 in spherical high density lipoprotein revealed by small angle neutron scattering. J Biol Chem. 2011;286:12495–508. - PMC - PubMed
-
- Anastasius M, Luquain-Costaz C, Kockx M, Jessup W and Kritharides L. A critical appraisal of the measurement of serum ‘cholesterol efflux capacity’ and its use as surrogate marker of risk of cardiovascular disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1257–1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

