Salmonella Typhimurium Adhesin OmpV Activates Host Immunity To Confer Protection against Systemic and Gastrointestinal Infection in Mice
- PMID: 34097470
- PMCID: PMC8281224
- DOI: 10.1128/IAI.00121-21
Salmonella Typhimurium Adhesin OmpV Activates Host Immunity To Confer Protection against Systemic and Gastrointestinal Infection in Mice
Abstract
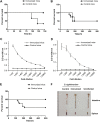
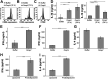
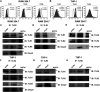
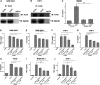
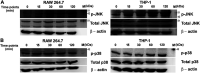
Salmonella enterica Typhimurium is a rod-shaped Gram-negative bacterium that mostly enters the human body through contaminated food. It causes a gastrointestinal disorder called salmonellosis in humans and typhoid-like systemic disease in mice. OmpV, an outer membrane protein of S. Typhimurium, helps in adhesion and invasion of bacteria to intestinal epithelial cells and thus plays a vital role in the pathogenesis of S. Typhimurium. In this study, we have shown that intraperitoneal immunization with OmpV is able to induce high IgG production and protection against systemic disease. Further, oral immunization with OmpV-incorporated proteoliposome (OmpV-proteoliposome [PL]) induces production of high IgA antibody levels and protection against gastrointestinal infection. Furthermore, we have shown that OmpV induces Th1 bias in systemic immunization with purified OmpV, but both Th1 and Th2 polarization in oral immunization with OmpV-proteoliposome (PL). Additionally, we have shown that OmpV activates innate immune cells, such as monocytes, macrophages, and intestinal epithelial cells, in a Toll-like receptor 2 (TLR2)-dependent manner. Interestingly, OmpV is recognized by the TLR1/2 heterodimer in monocytes, but by both TLR1/2 and TLR2/6 heterodimers in macrophages and intestinal epithelial cells. Further, downstream signaling involves MyD88, interleukin-1 receptor-associated kinase (IRAK)-1, mitogen-activated protein kinase (MAPK) (both p38 and Jun N-terminal protein kinase (JNK)), and transcription factors NF-κB and AP-1. Due to its ability to efficiently activate both the innate and adaptive immune systems and protective efficacy, OmpV can be a potential vaccine candidate against S. Typhimurium infection. Further, the fact that OmpV can be recognized by both TLR1/2 and TLR2/6 heterodimers increases its potential to act as good adjuvant in other vaccine formulations.
Keywords: OmpV; Salmonella; immune mechanisms.
Figures




Similar articles
-
Outer membrane protein OmpV mediates Salmonella enterica serovar typhimurium adhesion to intestinal epithelial cells via fibronectin and α1β1 integrin.Cell Microbiol. 2020 May;22(5):e13172. doi: 10.1111/cmi.13172. Epub 2020 Feb 10. Cell Microbiol. 2020. PMID: 32017350
-
Innate immunity mediated by MyD88 signal is not essential for induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection.J Immunol. 2009 Feb 15;182(4):2305-12. doi: 10.4049/jimmunol.0801980. J Immunol. 2009. PMID: 19201885 Free PMC article.
-
Innate immune detection of flagellin positively and negatively regulates salmonella infection.PLoS One. 2013 Aug 19;8(8):e72047. doi: 10.1371/journal.pone.0072047. eCollection 2013. PLoS One. 2013. PMID: 23977202 Free PMC article.
-
Innate immune response to Salmonella typhimurium, a model enteric pathogen.Gut Microbes. 2012 Mar-Apr;3(2):62-70. doi: 10.4161/gmic.19141. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22198618 Free PMC article. Review.
-
Evasion of host immunity by virulent Salmonella: implications for vaccine design.Curr Med Chem. 2011;18(36):5666-75. doi: 10.2174/092986711798347333. Curr Med Chem. 2011. PMID: 22172071 Review.
Cited by
-
A Potential Adhesin/Invasin STM0306 Participates in Host Cell Inflammation Induced by Salmonella enterica Serovar Typhimurium.Int J Mol Sci. 2023 May 3;24(9):8170. doi: 10.3390/ijms24098170. Int J Mol Sci. 2023. PMID: 37175877 Free PMC article.
-
Vibrio cholerae cytolysin induces pro-inflammatory and death signals through novel TLR assembly.PLoS Pathog. 2025 Apr 4;21(4):e1013033. doi: 10.1371/journal.ppat.1013033. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40184418 Free PMC article.
-
Comparative Reverse Vaccinology of Piscirickettsia salmonis, Aeromonas salmonicida, Yersinia ruckeri, Vibrio anguillarum and Moritella viscosa, Frequent Pathogens of Atlantic Salmon and Lumpfish Aquaculture.Vaccines (Basel). 2022 Mar 18;10(3):473. doi: 10.3390/vaccines10030473. Vaccines (Basel). 2022. PMID: 35335104 Free PMC article.
-
Comparative secretomic and proteomic analysis reveal multiple defensive strategies developed by Vibrio cholerae against the heavy metal (Cd2+, Ni2+, Pb2+, and Zn2+) stresses.Front Microbiol. 2023 Oct 26;14:1294177. doi: 10.3389/fmicb.2023.1294177. eCollection 2023. Front Microbiol. 2023. PMID: 37954246 Free PMC article.
-
Vibrio cholerae senses human enteric α-defensin 5 through a CarSR two-component system to promote bacterial pathogenicity.Commun Biol. 2022 Jun 8;5(1):559. doi: 10.1038/s42003-022-03525-3. Commun Biol. 2022. PMID: 35676416 Free PMC article.
References
-
- Alghoribi MF, Doumith M, Alrodayyan M, Al Zayer M, Koster WL, Muhanna A, Aljohani SM, Balkhy HH, Desin TS. 2019. S. Enteritidis and S. Typhimurium harboring SPI-1 and SPI-2 are the predominant serotypes associated with human salmonellosis in Saudi Arabia. Front Cell Infect Microbiol 9:187. 10.3389/fcimb.2019.00187. - DOI - PMC - PubMed
-
- Sparham SJ, Kwong JC, Valcanis M, Easton M, Trott DJ, Seemann T, Stinear TP, Howden BP. 2017. Emergence of multidrug resistance in locally-acquired human infections with Salmonella Typhimurium in Australia owing to a new clade harbouring blaCTX-M-9. Int J Antimicrob Agents 50:101–105. 10.1016/j.ijantimicag.2017.02.014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

