Toward New Transmission-Blocking Combination Therapies: Pharmacokinetics of 10-Amino-Artemisinins and 11-Aza-Artemisinin and Comparison with Dihydroartemisinin and Artemether
- PMID: 34097488
- PMCID: PMC8284440
- DOI: 10.1128/AAC.00990-21
Toward New Transmission-Blocking Combination Therapies: Pharmacokinetics of 10-Amino-Artemisinins and 11-Aza-Artemisinin and Comparison with Dihydroartemisinin and Artemether
Abstract
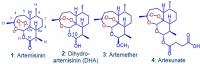
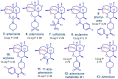
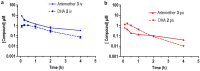
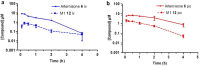
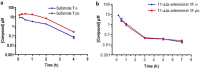
As artemisinin combination therapies (ACTs) are compromised by resistance, we are evaluating triple combination therapies (TACTs) comprising an amino-artemisinin, a redox drug, and a third drug with a different mode of action. Thus, here we briefly review efficacy data on artemisone, artemiside, other amino-artemisinins, and 11-aza-artemisinin and conduct absorption, distribution, and metabolism and excretion (ADME) profiling in vitro and pharmacokinetic (PK) profiling in vivo via intravenous (i.v.) and oral (p.o.) administration to mice. The sulfamide derivative has a notably long murine microsomal half-life (t1/2 > 150 min), low intrinsic liver clearance and total plasma clearance rates (CLint 189.4, CLtot 32.2 ml/min/kg), and high relative bioavailability (F = 59%). Kinetics are somewhat similar for 11-aza-artemisinin (t1/2 > 150 min, CLint = 576.9, CLtot = 75.0 ml/min/kg), although bioavailability is lower (F = 14%). In contrast, artemether is rapidly metabolized to dihydroartemisinin (DHA) (t1/2 = 17.4 min) and eliminated (CLint = 855.0, CLtot = 119.7 ml/min/kg) and has low oral bioavailability (F) of 2%. While artemisone displays low t1/2 of <10 min and high CLint of 302.1, it displays a low CLtot of 42.3 ml/min/kg and moderate bioavailability (F) of 32%. Its active metabolite M1 displays a much-improved t1/2 of >150 min and a reduced CLint of 37.4 ml/min/kg. Artemiside has t1/2 of 12.4 min, CLint of 673.9, and CLtot of 129.7 ml/kg/min, likely a reflection of its surprisingly rapid metabolism to artemisone, reported here for the first time. DHA is not formed from any amino-artemisinin. Overall, the efficacy and PK data strongly support the development of selected amino-artemisinins as components of new TACTs.
Keywords: amino-artemisinins; antimalarial agents; combination therapies; pharmacokinetics; transmission-blocking.
Figures


References
-
- World Health Organization. 2019. Geneva: world malaria report 2019.
-
- Hamilton WL, Amato R, van der Pluijm RW, Jacob CG, Quang HH, Thuy-Nhien NT, Hien TT, Hongvanthong B, Chindavongsa K, Mayxay M, Huy R, Leang R, Huch C, Dysoley L, Amaratunga C, Suon S, Fairhurst RM, Tripura R, Peto TJ, Sovann Y, Jittamala P, Hanboonkunupakarn B, Pukrittayakamee S, Chau NH, Imwong M, Dhorda M, Vongpromek R, Chan XHS, Maude RJ, Pearson RD, Nguyen T, Rockett K, Drury E, Gonçalves S, White NJ, Day NP, Kwiatkowski DP, Dondorp AM, Miotto O. 2019. Evolution and expansion of multidrug resistant malaria in Southeast Asia: a genomic epidemiology study. Lancet Infect Dis 19:943–951. 10.1016/S1473-3099(19)30392-5. - DOI - PMC - PubMed
-
- Witmer K, Dahalan FA, Delves MJ, Yahiya S, Watson OJ, Straschil U, Chiwcharoen D, Sornboon B, Pukrittayakamee S, Pearson RD, Howick VM, Lawniczak MKN, White NJ, Dondorp AM, Okell LC, Chotivanich K, Ruecker A, Baum J. 2020. Transmission of artemisinin-resistant malaria parasites to mosquitoes under antimalarial drug pressure. Antimicrob Agents Chemother 65:e00898-20. 10.1128/AAC.00898-20. - DOI - PMC - PubMed
-
- Miotto O, Sekihara M, Tachibana S-I, Yamauchi M, Pearson RD, Amato R, Gonçalves S, Mehra S, Noviyanti R, Marfurt J, Auburn S, Price RN, Mueller I, Ikeda M, Mori T, Hirai M, Tavul L, Hetzel MW, Laman M, Barry AE, Ringwald P, Ohashi J, Hombhanje F, Kwiatkowski DP, Mita T. 2020. Emergence of artemisinin-resistant Plasmodium falciparum with kelch13 C580Y mutations on the island of New Guinea. PLoS Pathog 16:e1009133. 10.1371/journal.ppat.1009133. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

