Calprotectin (S100A8/A9) Is an Innate Immune Effector in Experimental Periodontitis
- PMID: 34097505
- PMCID: PMC8445179
- DOI: 10.1128/IAI.00122-21
Calprotectin (S100A8/A9) Is an Innate Immune Effector in Experimental Periodontitis
Abstract
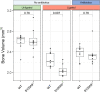
Upregulated in inflammation, calprotectin (complexed S100A8 and S100A9; S100A8/A9) functions as an innate immune effector molecule, promoting inflammation, and also as an antimicrobial protein. We hypothesized that antimicrobial S100A8/A9 would mitigate change to the local microbial community and promote resistance to experimental periodontitis in vivo. To test this hypothesis, S100A9-/- and wild-type (WT; S100A9+/+) C57BL/6 mice were compared using a model of ligature-induced periodontitis. On day 2, WT mice showed fewer infiltrating innate immune cells than S100A9-/- mice; by day 5, the immune cell numbers were similar. At 5 days post ligature placement, oral microbial communities sampled with swabs differed significantly in beta diversity between the mouse genotypes. Ligatures recovered from molar teeth of S100A9-/- and WT mice contained significantly dissimilar microbial genera from each other and the overall oral communities from swabs. Concomitantly, the S100A9-/- mice had significantly greater alveolar bone loss than WT mice around molar teeth in ligated sites. When the oral microflora was ablated by antibiotic pretreatment, differences disappeared between WT and S100A9-/- mice in their immune cell infiltrates and alveolar bone loss. Calprotectin, therefore, suppresses emergence of a dysbiotic, proinflammatory oral microbial community, which reduces innate immune effector activity, including early recruitment of innate immune cells, mitigating subsequent alveolar bone loss and protecting against experimental periodontitis.
Keywords: calprotectin; experimental periodontitis; inflammation; innate immunity; microbiome.
Figures


References
-
- Kitamoto S, Nagao-Kitamoto H, Jiao Y, GillillandMG, 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N. 2020. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182:447–462. 10.1016/j.cell.2020.05.048. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

