A human antibody of potent efficacy against SARS-CoV-2 in rhesus macaques showed strong blocking activity to B.1.351
- PMID: 34097570
- PMCID: PMC8189090
- DOI: 10.1080/19420862.2021.1930636
A human antibody of potent efficacy against SARS-CoV-2 in rhesus macaques showed strong blocking activity to B.1.351
Abstract
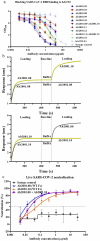
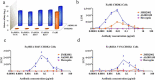
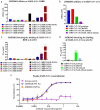
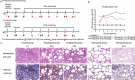
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes coronavirus disease-2019 (COVID-19), interacts with the host cell receptor angiotensin-converting enzyme 2 (hACE2) via its spike 1 protein during infection. After the virus sequence was published, we identified two potent antibodies against the SARS-CoV-2 receptor binding domain (RBD) from antibody libraries using a phage-to-yeast (PtY) display platform in only 10 days. Our lead antibody JMB2002, now in a Phase 1 clinical trial (ChiCTR2100042150), showed broad-spectrum in vitro blocking activity against hACE2 binding to the RBD of multiple SARS-CoV-2 variants, including B.1.351 that was reportedly much more resistant to neutralization by convalescent plasma, vaccine sera and some clinical-stage neutralizing antibodies. Furthermore, JMB2002 has demonstrated complete prophylactic and potent therapeutic efficacy in a rhesus macaque disease model. Prophylactic and therapeutic countermeasure intervention of SARS-CoV-2 using JMB2002 would likely slow down the transmission of currently emerged SARS-CoV-2 variants and result in more efficient control of the COVID-19 pandemic.
Keywords: B.1.1.7; B.1.351; D614G; JMB2002; SARS-CoV-2; broad-spectrum; neutralizing antibody; phage-to-yeast; rhesus macaque disease model.
Figures

References
-
- Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A, Slayton RB, Tong SX, Silk BJ, Armstrong GL, et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage-United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(3):95–99. doi: 10.15585/mmwr.mm7003e2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
