Analogous computations in working memory input, output and motor gating: Electrophysiological and computational modeling evidence
- PMID: 34097689
- PMCID: PMC8211210
- DOI: 10.1371/journal.pcbi.1008971
Analogous computations in working memory input, output and motor gating: Electrophysiological and computational modeling evidence
Abstract
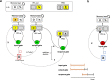
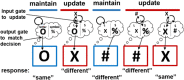
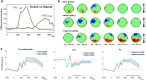
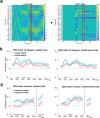
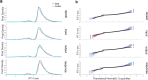
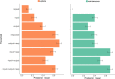
Adaptive cognitive-control involves a hierarchical cortico-striatal gating system that supports selective updating, maintenance, and retrieval of useful cognitive and motor information. Here, we developed a task that independently manipulates selective gating operations into working-memory (input gating), from working-memory (output gating), and of responses (motor gating) and tested the neural dynamics and computational principles that support them. Increases in gating demands, captured by gate switches, were expressed by distinct EEG correlates at each gating level that evolved dynamically in partially overlapping time windows. Further, categorical representations of specific maintained items and of motor responses could be decoded from EEG when the corresponding gate was switching, thereby linking gating operations to prioritization. Finally, gate switching at all levels was related to increases in the motor decision threshold as quantified by the drift diffusion model. Together these results support the notion that cognitive gating operations scaffold on top of mechanisms involved in motor gating.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Dreisbach G, Fröber K. On how to be flexible (or not): Modulation of the stability-flexibility balance. Current Directions in Psychological Science. 2019; 28: 3–9.
-
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. Control of cognitive processes: Attention and performance XVIII. 2000: 713–737.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

