Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis
- PMID: 34097769
- PMCID: PMC8208889
- DOI: 10.1002/14651858.CD012972.pub2
Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis
Abstract
Background: The World Health Organization (WHO) recommends Xpert MTB/RIF in place of smear microscopy to diagnose tuberculosis (TB), and many countries have adopted it into their diagnostic algorithms. However, it is not clear whether the greater accuracy of the test translates into improved health outcomes.
Objectives: To assess the impact of Xpert MTB/RIF on patient outcomes in people being investigated for tuberculosis.
Search methods: We searched the following databases, without language restriction, from 2007 to 24 July 2020: Cochrane Infectious Disease Group (CIDG) Specialized Register; CENTRAL; MEDLINE OVID; Embase OVID; CINAHL EBSCO; LILACS BIREME; Science Citation Index Expanded (Web of Science), Social Sciences citation index (Web of Science), and Conference Proceedings Citation Index - Social Science & Humanities (Web of Science). We also searched the WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, and the Pan African Clinical Trials Registry for ongoing trials.
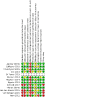
Selection criteria: We included individual- and cluster-randomized trials, and before-after studies, in participants being investigated for tuberculosis. We analysed the randomized and non-randomized studies separately. DATA COLLECTION AND ANALYSIS: For each study, two review authors independently extracted data, using a piloted data extraction tool. We assessed the risk of bias using Cochrane and Effective Practice and Organisation of Care (EPOC) tools. We used random effects meta-analysis to allow for heterogeneity between studies in setting and design. The certainty of the evidence in the randomized trials was assessed by GRADE.
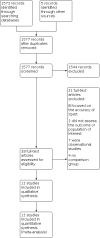
Main results: We included 12 studies: eight were randomized controlled trials (RCTs), and four were before-and-after studies. Most included RCTs had a low risk of bias in most domains of the Cochrane 'Risk of bias' tool. There was inconclusive evidence of an effect of Xpert MTB/RIF on all-cause mortality, both overall (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.75 to 1.05; 5 RCTs, 9932 participants, moderate-certainty evidence), and restricted to studies with six-month follow-up (RR 0.98, 95% CI 0.78 to 1.22; 3 RCTs, 8143 participants; moderate-certainty evidence). There was probably a reduction in mortality in participants known to be infected with HIV (odds ratio (OR) 0.80, 95% CI 0.67 to 0.96; 5 RCTs, 5855 participants; moderate-certainty evidence). It is uncertain whether Xpert MTB/RIF has no or a modest effect on the proportion of participants starting tuberculosis treatment who had a successful treatment outcome (OR) 1.10, 95% CI 0.96 to 1.26; 3RCTs, 4802 participants; moderate-certainty evidence). There was also inconclusive evidence of an effect on the proportion of participants who were treated for tuberculosis (RR 1.10, 95% CI 0.98 to 1.23; 5 RCTs, 8793 participants; moderate-certainty evidence). The proportion of participants treated for tuberculosis who had bacteriological confirmation was probably higher in the Xpert MTB/RIF group (RR 1.44, 95% CI 1.29 to 1.61; 6 RCTs, 2068 participants; moderate-certainty evidence). The proportion of participants with bacteriological confirmation who were lost to follow-up pre-treatment was probably reduced (RR 0.59, 95% CI 0.41 to 0.85; 3 RCTs, 1217 participants; moderate-certainty evidence).
Authors' conclusions: We were unable to confidently rule in or rule out the effect on all-cause mortality of using Xpert MTB/RIF rather than smear microscopy. Xpert MTB/RIF probably reduces mortality among participants known to be infected with HIV. We are uncertain whether Xpert MTB/RIF has a modest effect or not on the proportion treated or, among those treated, on the proportion with a successful outcome. It probably does not have a substantial effect on these outcomes. Xpert MTB/RIF probably increases both the proportion of treated participants who had bacteriological confirmation, and the proportion with a laboratory-confirmed diagnosis who were treated. These findings may inform decisions about uptake alongside evidence on cost-effectiveness and implementation.
Antecedentes: La Organización Mundial de la Salud (OMS) recomienda la Xpert MTB/RIF en lugar de la baciloscopia para diagnosticar la tuberculosis (TB) y muchos países la han adoptado en sus algoritmos de diagnóstico. Sin embargo, no está claro si la mayor exactitud de la prueba se traduce en mejores desenlaces de salud.
Objetivos: Evaluar el impacto de la Xpert MTB/RIF en los desenlaces de las personas sometidas a pruebas para la tuberculosis. MÉTODOS DE BÚSQUEDA: Se realizaron búsquedas en las siguientes bases de datos, sin restricción de idioma, desde 2007 hasta el 24 de julio de 2020: Registro especializado del Grupo Cochrane de Enfermedades infecciosas (Cochrane Infectious Disease Group [CIDG]); CENTRAL; MEDLINE OVID; Embase OVID; CINAHL EBSCO; LILACS BIREME; Science Citation Index Expanded (Web of Science), Social Sciences citation index (Web of Science), y Conference Proceedings Citation Index ‐ Social Science & Humanities (Web of Science). También se buscaron ensayos en curso en la Plataforma de registros internacionales de ensayos clínicos de la OMS, en ClinicalTrials.gov y en el Pan African Clinical Trials Registry. CRITERIOS DE SELECCIÓN: Se incluyeron ensayos aleatorizados individuales y por conglomerados, y estudios tipo antes y después (before‐after studie), con participantes sometidos a pruebas para la tuberculosis. Los estudios aleatorizados y no aleatorizados se analizaron por separado. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión, de forma independiente, extrajeron los datos de cada estudio mediante una herramienta de extracción de datos analizada. El riesgo de sesgo se evaluó mediante las herramientas de Cochrane y del Grupo Cochrane para una Práctica y organización sanitarias efectivas (Effective Practice and Organisation of Care [EPOC]). Se utilizó el metanálisis de efectos aleatorios para considerar la heterogeneidad entre los estudios en cuanto al contexto y el diseño. La certeza de la evidencia en los ensayos aleatorizados se evaluó mediante el método GRADE.
Resultados principales: Se incluyeron 12 estudios: ocho eran ensayos controlados aleatorizados (ECA) y cuatro eran estudios tipo antes y después. La mayoría de los ECA incluidos tenían un bajo riesgo de sesgo en la mayoría de los dominios de la herramienta Cochrane "Risk of bias". Hubo evidencia no concluyente de un efecto de la Xpert MTB/RIF sobre la mortalidad por todas las causas, tanto en general (razón de riesgos [RR] 0,89; intervalo de confianza [IC] del 95%: 0,75 a 1,05; cinco ECA, 9932 participantes, evidencia de certeza moderada), como limitada a los estudios con seguimiento de seis meses (RR 0,98; IC del 95%: 0,78 a 1,22; tres ECA, 8143 participantes; evidencia de certeza moderada). Probablemente hubo una reducción de la mortalidad en los participantes que se sabía que estaban infectados por el VIH (odds ratio [OR] 0,80; IC del 95%: 0,67 a 0,96; cinco ECA, 5855 participantes; evidencia de certeza moderada). No está claro si la Xpert MTB/RIF no tiene efectos o tiene un efecto modesto sobre la proporción de participantes que inician el tratamiento de la tuberculosis y que tienen un desenlace exitoso del tratamiento (OR 1,10; IC del 95%: 0,96 a 1,26; tres ECA, 4802 participantes; evidencia de certeza moderada). También hubo evidencia no concluyente de un efecto sobre el porcentaje de participantes que recibieron tratamiento para la tuberculosis (RR 1,10; IC del 95%: 0,98 a 1,23; cinco ECA, 8793 participantes; evidencia de certeza moderada). Es probable que la proporción de participantes tratados por tuberculosis que tuvieron confirmación bacteriológica fuera mayor en el grupo de Xpert MTB/RIF (RR 1,44; IC del 95%: 1,29 a 1,61; seis ECA, 2068 participantes; evidencia de certeza moderada). Es probable que se redujera la proporción de participantes con confirmación bacteriológica que se perdió durante el seguimiento previo al tratamiento (RR 0,59; IC del 95%: 0,41 a 0,85; tres ECA, 1217 participantes; evidencia de certeza moderada).
Conclusiones de los autores: No fue posible descartar con seguridad el efecto sobre la mortalidad por todas las causas del uso de Xpert MTB/RIF en lugar de la baciloscopia. La Xpert MTB/RIF probablemente reduce la mortalidad en los participantes que se sabe que están infectados por el VIH. No hay certeza con respecto a si la Xpert MTB/RIF tiene un efecto modesto o no en la proporción tratada o, entre los tratados, en la proporción con un desenlace exitoso. Probablemente no tenga un efecto importante sobre estos desenlaces. La Xpert MTB/RIF probablemente aumenta la proporción de participantes tratados que tenían confirmación bacteriológica, así como la de aquellos con un diagnóstico confirmado por el laboratorio que fueron tratados. Estos hallazgos podrían servir de base para las decisiones sobre la adopción de la prueba, junto con la evidencia sobre la coste‐efectividad y la aplicación.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
FH has no known conflict of interest.
RRN has no known conflict of interest.
SGS is employed by the Foundation for Innovative New Diagnostics (FIND). FIND has conducted studies and published on Xpert MTB/RIF as part of a collaborative project between FIND, a Swiss non‐profit, Cepheid, a US company, and academic partners. The product developed through this partnership was developed under a contract that obligated FIND to pay for development costs and trial costs, and Cepheid to make the test available at preferential pricing to the public sector in developing countries. In addition, FIND conducted studies for the Xpert MTB/RIF Ultra assay, which have also been published.
MK has no known conflict of interest.
CMD was also employed by FIND during this review.
SG has no known conflict of interest.
KR is a board member of the Trial Safety Board, iM4TB, Lausanne, CH for a tuberculosis drug trial unrelated to the submitted work.
AR has no known conflict of interest.
Figures











Update of
References
References to studies included in this review
Agizew 2019a {published data only}
-
- Agizew T, Chihota V, Nyirenda S, Tedla Z, Auld AF, Mathebula U, et al. Tuberculosis treatment outcomes among people living with HIV diagnosed using Xpert MTB/RIF versus sputum-smear microscopy in Botswana: a stepped-wedge cluster randomised trial. BMC Infectious Diseases 2019;19:1058. - PMC - PubMed
Calligaro 2015 {published data only}
-
- Calligaro GL, Theron G, Khalfey H, Peter J, Meldau R, Matinyenya B, et al. Burden of tuberculosis in intensive care units in Cape Town, South Africa, and assessment of the accuracy and effect on patient outcomes of the Xpert MTB/RIF test on tracheal aspirate samples for diagnosis of pulmonary tuberculosis: a prospective burden of disease study with a nested randomised controlled trial. Lancet Respiratory Medicine 2015;3:621-30. - PubMed
Churchyard 2015 {published data only}
-
- Churchyard GJ, Stevens WS, Mametja KM, Chihota V, Nicol MP, Erasmus LK, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet. Global Health 2015;3:e450-7. - PubMed
Cox 2014 {published data only}
Di Tanna 2019 {published data only}
Durovni 2014 {published data only}
Mupfumi 2014 {published data only}
Ngwira 2019 {published data only}
-
- Ngwira LG, Corbett EL, Khundi M, Barnes GL, Nkhoma A, Murowa M, et al. Screening for tuberculosis with Xpert MTB/RIF assay versus fluorescent microscopy among adults newly diagnosed with human immunodeficiency virus in rural Malawi: a cluster randomised trial (Chepetsa). Clinical Infectious Diseases 2019;68(7):1176-83. [DOI: 10.1093/cid/ciy590] [PMID: ] - DOI - PMC - PubMed
Schmidt 2017 {published data only}
-
- Schmidt B-M, Geldenhuys H, Tameris M, Luabeya A, Mulenga H, Bunyasi E, et al. Impact of Xpert MTB/RIF rollout on management of tuberculosis in a South African community. South African Medical Journal 2017;107(12):1078-81. - PubMed
Theron 2014a {published data only}
-
- Theron G, Zijenah L, Chandra D, Clowes P, Rachow A, Lesoky M, et al, for the TB-NEAT Team. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383:424-35. - PubMed
Van den Handel 2015 {published data only}
-
- Van den Handel T, Hampton K, Sanne I, Stevens W, Croux R, Van Rie A. The impact of Xpert MTB/RIF in sparsely populated rural settings. International Journal of Tuberculosis and Lung Disease 2015;19(4):392-8. - PubMed
van Kampen 2015 {published data only}
References to studies excluded from this review
Boehme 2011 {published data only}
-
- Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377(9776):1495-505. - PMC - PubMed
Buchelli Ramirez 2014 {published data only}
-
- Buchelli Ramirez HL, García-Clemente MM, Alvarez-Álvarez C, Palacio-Gutierrez JJ, Pando-Sandoval A, Gagatek S, et al. Impact of the Xpert(®) MTB/RIF molecular test on the late diagnosis of pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2014;18(4):435-7. [DOI: 10.5588/ijtld.13.0747] [PMID: ] - DOI - PubMed
Chilembo 2020 {published data only}
Feasey 2013 {published data only}
-
- Feasey NA, Banada PP, Howson W, Sloan DJ, Mdolo A, Boehme C, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. Journal of Clinical Microbiology 2013;51(7):2311-6. [DOI: 10.1128/JCM.00330-13] [PMID: ] - DOI - PMC - PubMed
Hanrahan 2013 {published data only}
-
- Hanrahan CF, Selibas K, Deery CB, Dansey H, Clouse K, Bassett J, et al. Time to treatment and patient outcomes among TB suspects screened by a single point of care Xpert MTB/RIF at primary care clinic in Johannesburg, South Africa. PloS One 2013;8(6):e65421. [PMID: 10.1371/journal.pone.0065421] [PMID: ] - DOI - PMC - PubMed
Hanrahan 2015 {published data only}
Kim 2015 {published data only}
Kwak 2013 {published data only}
Lawn 2011 {published data only}
-
- Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Medicine 2011;8(7):e1001067. [DOI: 10.1371/journal.pmed.1001067] [PMID: ] - DOI - PMC - PubMed
Lebina 2016 {published data only}
Lessells 2017 {published data only}
-
- Lessells RJ, Cooke GS, McGrath N, Nicol MP, Newell ML, Godfrey-Faussett. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation: a cluster-randomized trial. American Journal of Respiratory and Critical Care Medicine 2017;196(7):901-10. [DOI: 10.1164/rccm.201702-0278OC] - DOI - PMC - PubMed
Mboze 2016 {published data only}
Metcalfe 2016 {published data only}
-
- Metcalfe JZ, Makumbirofa S, Makamure B, Sandy C, Bara W, Mason P, et al. Xpert MTB/RIF detection of rifampicin resistance and time to treatment initiation in Harare, Zimbabwe. International Journal of Tuberculosis and Lung Disease 2016;20(7):882-9. [DOI: 10.5588/ijtld.15.0696] [PMID: ] - DOI - PMC - PubMed
Mwansa‐Kambafwile 2016 {published data only}
O'Grady 2012 {published data only}
-
- O'Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clinical Infectious Diseases 2012;55(9):1171-8. [PMID: 10.1093/cid/cis631] [PMID: ] - DOI - PubMed
Padayatchi 2016 {published data only}
-
- Padayatchi N, Naidu N, Yende-Zuma N, Roe O’Donnell M, Naidoo K, Augustine S, et al. Implementation and Operational Research: clinical impact of the Xpert MTB/RIF assay in patients with multidrug-resistant tuberculosis. Journal of Acquired Immune Deficiency Syndromes 2016;73(1):e1-7. - PubMed
Rachow 2011 {published data only}
Sachdeva 2015 {published data only}
-
- Sachdeva KS, Raizada N, Sreenivas A, Van't Hoog AH, den Hof S, Dewan PK, et al. Use of Xpert MTB/RIF in decentralized public health settings and its effect on pulmonary TB and DR-TB case finding in India. PloS One 2015;10(5):e0126065. [DOI: 10.1371/journal.pone.0126065] [PMID: ] - DOI - PMC - PubMed
Scott 2010 {published data only}
-
- Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, et al. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: a prospective study. PLoS Medicine 2011;8(7):e1001061. [DOI: 10.1371/journal.pmed.1001061] [PMID: ] - DOI - PMC - PubMed
Theron 2011 {published data only}
-
- Theron G, Peter J, Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for diagnosing of pulmonary tuberculosis in a high prevalence setting. American Journal of Respiratory and Critical Care Medicine 2011;184(1):132-40. [DOI: 10.1164/rccm.201101-0056OC] [PMID: ] - DOI - PubMed
Wang 2020 {published data only}
-
- Wang P, Gu J, Yang J, Yang C, Wu X, Yu F, et al. Effect of Xpert MTB/RIF on the treatment of multi-drug-resistant or rifampicin-resistant tuberculosis screened out from re-treatment pulmonary tuberculosis patients, a prospective cohort study. Annals of Palliative Medicine 2020;9(2):239-46. - PubMed
Additional references
Agizew 2019b
-
- Agizew T, Boyd R, Auld AF, Payton L, Pals SL, Lekone P, et al. Treatment outcomes, diagnostic and therapeutic impact: Xpert vs. smear. A systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease 2019;23(1):82-92. [DOI: 10.5588/ijtld.18.0203] [PMID: ] - DOI - PMC - PubMed
Albert 2016
Auld 2016
-
- Auld AF, Fielding KL, Gupta-Wright A, Lawn SD. Xpert MTB/RIF - why the lack of morbidity and mortality impact in intervention trials? Transactions of the Royal Society of Tropical Medicine and Hygiene 2016;110(8):432-44. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;54(4):401-6. - PubMed
Boehme 2010
Boyles 2017
Cook 2008
-
- Cook GC, Zumla A. Manson's Tropical Diseases. 22nd edition. Philadelphia: Saunders Ltd, 2008.
Corbett 2006
-
- Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367(9514):926-37. - PubMed
Creswell 2014
Creswell 2015
-
- Creswell J, Rai B, Wali R, Sudrungrot S, Adhikari LM, Pant R, et al. Introducing new tuberculosis diagnostics: the impact of Xpert MTB/RIF testing on case notifications in Nepal. International Journal of Tuberculosis and Lung Disease 2015;19(5):545-51. [DOI: 10.5588/ijtld.14.0775] [PMID: ] - DOI - PubMed
Denkinger 2014
-
- Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2014;44(2):435-46. - PubMed
Di Ruffano 2017a
-
- di Ruffano LF, Dinnes J, Sitch AJ, Hyde C, Deeks JJ. Test-treatment RCTs are susceptible to bias: a review of the methodological quality of randomized trials that evaluate diagnostic tests. BMC Medical Research Methodology 2017;17(35):35. [DOI: 10.1186/s12874-016-0287-z] [PMID: ] - DOI - PMC - PubMed
Di Ruffano 2017b
EPOC 2018
-
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews 2018. Available at epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 30 November 2020).
GRADE 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5360. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380-2. - PubMed
Helb 2010
Hermans 2017
-
- Hermans S, Caldwell J, Kaplan R, Cobelens F, Wood R. The impact of the roll-out of rapid molecular diagnostic testing for tuberculosis on empirical treatment in Cape Town, South Africa. Bulletin of the World Health Organization 2017;95(8):554-63. [DOI: 10.2471/BLT.16.185314] [PMID: ] - DOI - PMC - PubMed
Higgins 2011
Higgins 2019
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Houben 2016
Jüni 2001
Levy 1989
-
- Levy H, Feldman C, Sacho H, Meulen H, Kallenbach J, Koornhof H. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest 1989;95(6):1193-7. - PubMed
MacPherson 2014
-
- MacPherson P, Houben RMGJ, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bulletin of the World Health Organization 2014;92(2):126-38. [PMID: 10.2471/BLT.13.124800] [PMID: ] - DOI - PMC - PubMed
McGrath 2020
-
- McGrath G, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biometrical Journal 2020;62:69-98. - PubMed
Nliwasa 2018
Ochodo 2019
-
- Ochodo EA, Kalema N, Schumacher S, Steingart K, Young T, Mallett S, et al. Variation in the observed effect of Xpert MTB/RIF testing for tuberculosis on mortality: a systematic review and analysis of trial design considerations. Wellcome Open Research 2019;4:173. [DOI: 10.12688/wellcomeopenres.15412.1] - DOI - PMC - PubMed
Page 2019
-
- Page MJ, HIggins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Pai 2018
Parsons 2011
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 1.22. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Schumacher 2016
Scott 2014
Subbaraman 2019
-
- Subbaraman R, Nathavitharana RR, Mayer KH, Satyanarayana S, Chadha VK, Arinaminpathy N, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Medicine 2019;16(2):e1002754. [PMID: 10.1371/journal.pmed.1002754] [PMID: ] - DOI - PMC - PubMed
Theron 2014b
-
- Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infectious Diseases 2014;14:527-32. - PubMed
Walusimbi 2013
WHO 2013
-
- World Health Organization (WHO). Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Policy update. apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf 2013. - PubMed
WHO 2014
-
- World Health Organization (WHO). Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations. Available at www.who.int/tb/publications/xpert_implem_manual/en/ 2014. - PubMed
WHO 2014b
-
- World Health Organization (WHO). The End TB strategy - Global strategy and targets for tuberculosis prevention, care and control after 2015. www.who.int/tb/strategy/End_TB_Strategy.pdf March 2014.
WHO 2016a
-
- World Health Organization (WHO). The use of molecular line probe assays for the detection of resistance to isoniazid and rifampicin. Available at www.who.int/tb/publications/molecular-test-resistance/en/ 2016.
WHO 2016b
-
- World Health Organization (WHO). WHO monitoring of Xpert MTB/RIF roll-out. Available at www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/ 2016.
WHO 2019
-
- World Health Organization (WHO). Global report for tuberculosis. Available at www.who.int/tb/publications/global_report/en/ 2019.
Zifodya 2021
-
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub5] - DOI - PMC - PubMed
References to other published versions of this review
Haraka 2018
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

