Experience-related enhancements in striatal temporal encoding
- PMID: 34097793
- PMCID: PMC8511940
- DOI: 10.1111/ejn.15344
Experience-related enhancements in striatal temporal encoding
Abstract
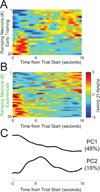
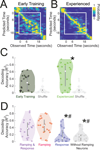
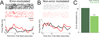
Temporal control of action is key for a broad range of behaviors and is disrupted in human diseases such as Parkinson's disease and schizophrenia. A brain structure that is critical for temporal control is the dorsal striatum. Experience and learning can influence dorsal striatal neuronal activity, but it is unknown how these neurons change with experience in contexts which require precise temporal control of movement. We investigated this question by recording from medium spiny neurons (MSNs) via dorsal striatal microelectrode arrays in mice as they gained experience controlling their actions in time. We leveraged an interval timing task optimized for mice which required them to "switch" response ports after enough time had passed without receiving a reward. We report three main results. First, we found that time-related ramping activity and response-related activity increased with task experience. Second, temporal decoding by MSN ensembles improved with experience and was predominantly driven by time-related ramping activity. Finally, we found that a subset of MSNs had differential modulation on error trials. These findings enhance our understanding of dorsal striatal temporal processing by demonstrating how MSN ensembles can evolve with experience. Our results can be linked to temporal habituation and illuminate striatal flexibility during interval timing, which may be relevant for human disease.
© 2021 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
Competing Interests
There are no conflicts of interest.
Figures


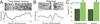
Similar articles
-
Rodent Medial Frontal Control of Temporal Processing in the Dorsomedial Striatum.J Neurosci. 2017 Sep 6;37(36):8718-8733. doi: 10.1523/JNEUROSCI.1376-17.2017. Epub 2017 Aug 8. J Neurosci. 2017. PMID: 28821670 Free PMC article.
-
Coordinated Ramping of Dorsal Striatal Pathways preceding Food Approach and Consumption.J Neurosci. 2018 Apr 4;38(14):3547-3558. doi: 10.1523/JNEUROSCI.2693-17.2018. Epub 2018 Mar 9. J Neurosci. 2018. PMID: 29523623 Free PMC article.
-
Ventrolateral Striatal Medium Spiny Neurons Positively Regulate Food-Incentive, Goal-Directed Behavior Independently of D1 and D2 Selectivity.J Neurosci. 2017 Mar 8;37(10):2723-2733. doi: 10.1523/JNEUROSCI.3377-16.2017. Epub 2017 Feb 6. J Neurosci. 2017. PMID: 28167674 Free PMC article.
-
Optogenetic insights into striatal function and behavior.Behav Brain Res. 2013 Oct 15;255:44-54. doi: 10.1016/j.bbr.2013.04.018. Epub 2013 Apr 28. Behav Brain Res. 2013. PMID: 23628212 Review.
-
Reappraising striatal D1- and D2-neurons in reward and aversion.Neurosci Biobehav Rev. 2016 Sep;68:370-386. doi: 10.1016/j.neubiorev.2016.05.021. Epub 2016 May 24. Neurosci Biobehav Rev. 2016. PMID: 27235078 Review.
Cited by
-
Glycolysis-enhancing α1-adrenergic antagonists modify cognitive symptoms related to Parkinson's disease.NPJ Parkinsons Dis. 2023 Mar 2;9(1):32. doi: 10.1038/s41531-023-00477-1. NPJ Parkinsons Dis. 2023. PMID: 36864060 Free PMC article.
-
Long-term, cell type-specific effects of prenatal stress on dorsal striatum and relevant behaviors in mice.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.627207. doi: 10.1101/2024.12.27.627207. bioRxiv. 2024. PMID: 39763907 Free PMC article. Preprint.
-
Sub-second and multi-second dopamine dynamics underlie variability in human time perception.medRxiv [Preprint]. 2024 Feb 9:2024.02.09.24302276. doi: 10.1101/2024.02.09.24302276. medRxiv. 2024. PMID: 38370629 Free PMC article. Preprint.
-
Complementary cognitive roles for D2-MSNs and D1-MSNs during interval timing.Elife. 2025 Jan 15;13:RP96287. doi: 10.7554/eLife.96287. Elife. 2025. PMID: 39812105 Free PMC article.
-
Alpha-synuclein pre-formed fibrils injected into prefrontal cortex primarily spread to cortical and subcortical structures and lead to isolated behavioral symptoms.bioRxiv [Preprint]. 2023 May 15:2023.01.31.526365. doi: 10.1101/2023.01.31.526365. bioRxiv. 2023. Update in: J Parkinsons Dis. 2024;14(1):81-94. doi: 10.3233/JPD-230129. PMID: 36778400 Free PMC article. Updated. Preprint.
References
-
- Balci F, Ludvig EA, Gibson JM, Allen BD, Frank KM, Kapustinski BJ, Fedolak TE, & Brunner D (2008) Pharmacological manipulations of interval timing using the peak procedure in male C3H mice. Psychopharmacology (Berl), 201, 67–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

