Light-Driven Carbene Catalysis for the Synthesis of Aliphatic and α-Amino Ketones
- PMID: 34097802
- PMCID: PMC8338790
- DOI: 10.1002/anie.202105354
Light-Driven Carbene Catalysis for the Synthesis of Aliphatic and α-Amino Ketones
Abstract
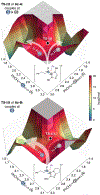
Single-electron N-heterocyclic carbene (NHC) catalysis has gained attention recently for the synthesis of C-C bonds. Guided by density functional theory and mechanistic analyses, we report the light-driven synthesis of aliphatic and α-amino ketones using single-electron NHC operators. Computational and experimental results reveal that the reactivity of the key radical intermediate is substrate-dependent and can be modulated through steric and electronic parameters of the NHC. Catalyst potential is harnessed in the visible-light driven generation of an acyl azolium radical species that undergoes selective coupling with various radical partners to afford diverse ketone products. This methodology is showcased in the direct late-stage functionalization of amino acids and pharmaceutical compounds, highlighting the utility of single-electron NHC operators.
Keywords: carbene; catalysis; density functional theory; late-stage functionalization; photochemistry.
© 2021 Wiley-VCH GmbH.
Figures
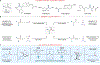

References
-
- Chen KK, Ann. N.Y. Acad. Sci 1948, 51, 83–97; - PubMed
- Boyle EA, Freemanm PC, Mangan FR, Thomson MJ, J. Pharm. Pharmacol 1982, 34, 562–569; - PubMed
- Tanaka T, Kawase M, Tani S, Biorg. Med. Chem. 2004, 12, 501–505; - PubMed
- Redelinghuys P, Nchinda AT, Chibale K, Sturrock ED, Biol. Chem 2006, 387, 461–466; - PubMed
- Riley AB, Tafreshi MJ, Haber SL, Am. J. Health-Syst. Pharm 2008, 65, 1019–1028; - PubMed
- Hoyos P, Sinisterra J-V, Molinari F, Alcántara AR, Domínguez de María P, Acc. Chem. Res 2010, 43, 288–299; - PubMed
- Liu Y, Han S-J, Liu W-B, Stoltz BM, Acc. Chem. Res 2015, 48, 740–751; - PMC - PubMed
- Allen LAT, Raclea R-C, Natho P, Parsons PJ, Org. Biomol. Chem 2021, 19, 498–513. - PubMed
-
- Milstein D, Stille JK, J. Am. Chem. Soc 1978, 100, 3636–3638;
- Richardson SK, in General and Synthetic Methods: Volume 14, Vol. 14 (Ed.: Pattenden G), The Royal Society of Chemistry, 1992, pp. 26–62;
- Dieter RK, Tetrahedron 1999, 55, 4177–4236;
- Zhou F, Li C-J, Nat. Commun 2014, 5, 4254; - PubMed
- Miles DH, Guasch J, Toste FD, J. Am. Chem. Soc 2015, 137, 7632–7635; - PMC - PubMed
- Zhang M, Xie J, Zhu C, Nat. Commun 2018, 9, 3517; - PMC - PubMed
- Ruzi R, Liu K, Zhu C, Xie J, Nat. Commun 2020, 11, 3312. - PMC - PubMed
-
- Grignard V, Hebd CR. Séances. Acad. Sci 1900, 130, 1322– 1325;
- Nahm S, Weinreb SM, Tetrahedron Lett. 1981, 22, 3815–3818;
- Alonso F, Lorenzo E, Yus M, J. Org. Chem 1996, 61, 6058–6059;
- Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA, Angew. Chem. Int. Ed 2003, 42, 4302–4320; - PubMed
- Kagan HB, Angew. Chem. Int. Ed 2012, 51, 7376–7382; - PubMed
- Colas K, dos Santos ACVD, Mendoza A, Org. Lett 2019, 21, 7908–7913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

