A deep-sea bacterium related to coastal marine pathogens
- PMID: 34097814
- PMCID: PMC8519021
- DOI: 10.1111/1462-2920.15629
A deep-sea bacterium related to coastal marine pathogens
Abstract
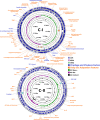
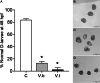
Evolution of virulence traits from adaptation to environmental niches other than the host is probably a common feature of marine microbial pathogens, whose knowledge might be crucial to understand their emergence and pathogenetic potential. Here, we report genome sequence analysis of a novel marine bacterial species, Vibrio bathopelagicus sp. nov., isolated from warm bathypelagic waters (3309 m depth) of the Mediterranean Sea. Interestingly, V. bathopelagicus sp. nov. is closely related to coastal Vibrio strains pathogenic to marine bivalves. V. bathopelagicus sp. nov. genome encodes genes involved in environmental adaptation to the deep-sea but also in virulence, such as the R5.7 element, MARTX toxin cluster, Type VI secretion system and zinc-metalloprotease, previously associated with Vibrio infections in farmed oysters. The results of functional in vitro assays on immunocytes (haemocytes) of the Mediterranean mussel Mytilus galloprovincialis and the Pacific oyster Crassostrea gigas, and of the early larval development assay in Mytilus support strong toxicity of V. bathopelagicus sp. nov. towards bivalves. V. bathopelagicus sp. nov., isolated from a remote Mediterranean bathypelagic site, is an example of a planktonic marine bacterium with genotypic and phenotypic traits associated with animal pathogenicity, which might have played an evolutionary role in the origin of coastal marine pathogens.
© 2021 The Authors. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures


References
-
- Aono, E. , Baba, T. , Ara, T. , Nishi, T. , Nakamichi, T. , Inamoto, E. , et al. (2010) Complete genome sequence and comparative analysis of Shewanella violacea, a psychrophilic and piezophilic bacterium from deep sea floor sediments. Mol Biosyst 6: 1216–1226. - PubMed
-
- ASTM (2004). Standard guide for conducting static acute toxicity tests starting with embryos of four species of saltwater bivalve molluscs. URL 10.1520/E0724-98. - DOI
-
- Balbi, T. , Auguste, M. , Cortese, K. , Montagna, M. , Borello, A. , Pruzzo, C. , et al. (2018a) Responses of Mytilus galloprovincialis to challenge with the emerging marine pathogen Vibrio coralliilyticus . Fish Shellfish Immunol 84: 352–360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

