Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial
- PMID: 34097856
- PMCID: PMC8177770
- DOI: 10.1016/S0140-6736(21)01203-4
Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial
Abstract
Background: COVID-19 is associated with a prothrombotic state leading to adverse clinical outcomes. Whether therapeutic anticoagulation improves outcomes in patients hospitalised with COVID-19 is unknown. We aimed to compare the efficacy and safety of therapeutic versus prophylactic anticoagulation in this population.
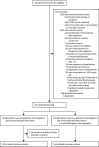
Methods: We did a pragmatic, open-label (with blinded adjudication), multicentre, randomised, controlled trial, at 31 sites in Brazil. Patients (aged ≥18 years) hospitalised with COVID-19 and elevated D-dimer concentration, and who had COVID-19 symptoms for up to 14 days before randomisation, were randomly assigned (1:1) to receive either therapeutic or prophylactic anticoagulation. Therapeutic anticoagulation was in-hospital oral rivaroxaban (20 mg or 15 mg daily) for stable patients, or initial subcutaneous enoxaparin (1 mg/kg twice per day) or intravenous unfractionated heparin (to achieve a 0·3-0·7 IU/mL anti-Xa concentration) for clinically unstable patients, followed by rivaroxaban to day 30. Prophylactic anticoagulation was standard in-hospital enoxaparin or unfractionated heparin. The primary efficacy outcome was a hierarchical analysis of time to death, duration of hospitalisation, or duration of supplemental oxygen to day 30, analysed with the win ratio method (a ratio >1 reflects a better outcome in the therapeutic anticoagulation group) in the intention-to-treat population. The primary safety outcome was major or clinically relevant non-major bleeding through 30 days. This study is registered with ClinicalTrials.gov (NCT04394377) and is completed.
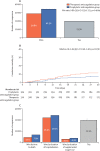
Findings: From June 24, 2020, to Feb 26, 2021, 3331 patients were screened and 615 were randomly allocated (311 [50%] to the therapeutic anticoagulation group and 304 [50%] to the prophylactic anticoagulation group). 576 (94%) were clinically stable and 39 (6%) clinically unstable. One patient, in the therapeutic group, was lost to follow-up because of withdrawal of consent and was not included in the primary analysis. The primary efficacy outcome was not different between patients assigned therapeutic or prophylactic anticoagulation, with 28 899 (34·8%) wins in the therapeutic group and 34 288 (41·3%) in the prophylactic group (win ratio 0·86 [95% CI 0·59-1·22], p=0·40). Consistent results were seen in clinically stable and clinically unstable patients. The primary safety outcome of major or clinically relevant non-major bleeding occurred in 26 (8%) patients assigned therapeutic anticoagulation and seven (2%) assigned prophylactic anticoagulation (relative risk 3·64 [95% CI 1·61-8·27], p=0·0010). Allergic reaction to the study medication occurred in two (1%) patients in the therapeutic anticoagulation group and three (1%) in the prophylactic anticoagulation group.
Interpretation: In patients hospitalised with COVID-19 and elevated D-dimer concentration, in-hospital therapeutic anticoagulation with rivaroxaban or enoxaparin followed by rivaroxaban to day 30 did not improve clinical outcomes and increased bleeding compared with prophylactic anticoagulation. Therefore, use of therapeutic-dose rivaroxaban, and other direct oral anticoagulants, should be avoided in these patients in the absence of an evidence-based indication for oral anticoagulation.
Funding: Coalition COVID-19 Brazil, Bayer SA.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JHA reports grants and personal fees from Bristol-Myers Squibb and CSL Behring; grants from AstraZeneca, CryoLife, US Food & Drug Administration, US National Institutes of Health, Sanofi, VoluMetrix, and Boehringer Ingelheim; personal fees from Pfizer, AbbVie Pharmaceuticals, Portola Pharmaceuticals, Quantum Genetics, Teikoku Pharmaceuticals, VA Cooperative Studies Program, and Zafgen, outside of the submitted work. AA reports consultant and lecture fees from Bayer, NovoNordisk, and LillyBaxter; lecture fees from Daichii-Sankyo; and research grants from Bayer, EMS Pharma, and the Population Health Research Institute, outside of the submitted work. LCPA reports personal fees from Baxter, Pfizer, and Halex-Istar; and grants from Ache Laboratorios Farmaceuticos, outside of the submitted work. OB reports grants from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Servier, and Amgen, and advisory board and personal fees from Novartis, outside of the submitted work. ABC reports grants from Bayer outside of the submitted work. GEC-S reports grants from Novartis and Air Liquide, outside of the submitted work. PGMdBeS reports grants from Bayer, Roche, and Pfizer, outside of the submitted work. MDAD reports personal fees, non-financial support, and other (advisory board participation) from Pfizer; personal fees and non-financial support from Bayer; personal fees and other (advisory board participation) from Servier; and personal fees from Boehringer Ingelheim, Daiichi Sankyo, and AstraZeneca, outside of the submitted work. RHMF reports grants from Bayer during the conduct of the study; and grants and personal fees from AstraZeneca and Servier, personal fees and non-financial support from Bayer, grants and non-financial support from EMS Pharma, and grants from Aché, Health Canada, and the Brazilian Ministry of Health, outside of the submitted work. MBG reports personal fees from COALITION COVID-19 Brazil and Bayer during the conduct of the study. RDL reports grants and personal fees from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim, outside of the submitted work. AVSM reports personal fees, non-financial support, and other (advisory board participation) from Bayer and Pfizer; personal fees and other (advisory board participation) from Novartis; personal fees and non-financial support from Zodiac; and personal fees from Ferring, Janssen, Sanofi, and AstraZeneca, outside of the submitted work. FCN reports grants and personal fees from Boehringer Ingelheim; and personal fees from Bayer and Pfizer, outside of the submitted work. ER reports grants and consulting fees from Bayer and Pfizer; grants from the Brazilian Ministry of Science and Technology; and personal fees from Aspen Pharma, Biomm Pharma, and Daiichi Sankyo, outside of the submitted work. ATR reports personal fees from Sanofi and Bayer, outside of the submitted work. VCV reports grants from Aspen Pharma, Pfizer, and Cristalia, outside of the submitted work. All other authors declare no competing interests.
Figures

Comment in
-
Anticoagulation in COVID-19: reaction to the ACTION trial.Lancet. 2021 Jun 12;397(10291):2226-2228. doi: 10.1016/S0140-6736(21)01291-5. Lancet. 2021. PMID: 34119049 Free PMC article. No abstract available.
-
In COVID-19, therapeutic vs. prophylactic anticoagulation did not improve clinical outcomes and increased bleeding.Ann Intern Med. 2021 Oct;174(10):JC112. doi: 10.7326/ACPJ202110190-112. Epub 2021 Oct 5. Ann Intern Med. 2021. PMID: 34606319
-
Age-adjusted D-dimer cutoffs to guide anticoagulation in COVID-19.Lancet. 2021 Oct 9;398(10308):1303-1304. doi: 10.1016/S0140-6736(21)01859-6. Lancet. 2021. PMID: 34627487 Free PMC article. No abstract available.
-
Age-adjusted D-dimer cutoffs to guide anticoagulation in COVID-19 - Authors' reply.Lancet. 2021 Oct 9;398(10308):1304. doi: 10.1016/S0140-6736(21)01909-7. Lancet. 2021. PMID: 34627488 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

