Foundational concepts in the biology of bacterial keratitis
- PMID: 34097906
- PMCID: PMC8595513
- DOI: 10.1016/j.exer.2021.108647
Foundational concepts in the biology of bacterial keratitis
Abstract
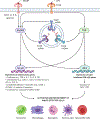
Bacterial infections of the cornea, or bacterial keratitis (BK), are notorious for causing rapidly fulminant disease and permanent vision loss, even among treated patients. In the last sixty years, dramatic upward trajectories in the frequency of BK have been observed internationally, driven in large part by the commercialization of hydrogel contact lenses in the late 1960s. Despite this worsening burden of disease, current evidence-based therapies for BK - including broad-spectrum topical antibiotics and, if indicated, topical corticosteroids - fail to salvage vision in a substantial proportion of affected patients. Amid growing concerns of rapidly diminishing antibiotic utility, there has been renewed interest in urgently needed novel treatments that may improve clinical outcomes on an individual and public health level. Bridging the translational gap in the care of BK requires the identification of new therapeutic targets and rational treatment design, but neither of these aims can be achieved without understanding the complex biological processes that determine how bacterial corneal infections arise, progress, and resolve. In this chapter, we synthesize the current wealth of human and animal experimental data that now inform our understanding of basic BK pathophysiology, in context with modern concepts in ocular immunology and microbiology. By identifying the key molecular determinants of clinical disease, we explore how novel treatments can be developed and translated into routine patient care.
Keywords: Adaptive immunity; Bacterial keratitis; Corneal infections; Immunology; Innate immunity; Microbial keratitis; Pseudomonas aeruginosa; Staphylococcus aureus; Streptococcus pneumoniae.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CONFLICTS OF INTEREST
None to declare.
Figures



References
-
- Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ, 2000. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 299, 39–46. - PubMed
-
- Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ, 1996. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest. Ophthalmol. Vis. Sci 37, 1826–1832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

