Atypical cytomegalovirus retinal disease in pyroptosis-deficient mice with murine acquired immunodeficiency syndrome
- PMID: 34097907
- PMCID: PMC8595509
- DOI: 10.1016/j.exer.2021.108651
Atypical cytomegalovirus retinal disease in pyroptosis-deficient mice with murine acquired immunodeficiency syndrome
Abstract
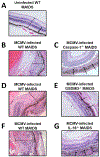
Pyroptosis is a caspase-dependent programmed cell death pathway that initiates and sustains inflammation through release of pro-inflammatory cytokines interleukin (IL)-1β and IL-18 following formation of gasdermin D (GSDMD)-mediated membrane pores. To determine the possible pathogenic contributions of pyroptosis toward development of full-thickness retinal necrosis during AIDS-related human cytomegalovirus retinitis, we performed a series of studies using an established model of experimental murine cytomegalovirus (MCMV) retinitis in mice with retrovirus-induced immunosuppression (MAIDS). Initial investigations demonstrated significant transcription and translation of key pyroptosis-associated genes within the ocular compartments of MCMV-infected eyes of mice with MAIDS. Subsequent investigations compared MCMV-infected eyes of groups of wildtype MAIDS mice with MCMV-infected eyes of groups of caspase-1-/- MAIDS mice, GSDMD-/- MAIDS mice, or IL-18-/- MAIDS mice to explore a possible contribution of pyroptosis towards the pathogenesis of MAIDS-related MCMV retinitis. Histopathologic analysis revealed typical full-thickness retinal necrosis in 100% of MCMV-infected eyes of wildtype MAIDS mice. In sharp contrast, none (0%) of MCMV-infected eyes of MAIDS mice that were deficient in either caspase-1, GSDMD, or IL-18 developed full-thickness retinal necrosis but instead exhibited an atypical pattern of retinal disease characterized by thickening and proliferation of the retinal pigmented epithelium layer with relative sparing of the neurosensory retina. Surprisingly, MCMV-infected eyes of all groups of deficient MAIDS mice harbored equivalent intraocular amounts of infectious virus as seen in MCMV-infected eyes of groups of wildtype MAIDS mice despite failure to develop full-thickness retinal necrosis. We conclude that pyroptosis plays a significant role in the development of full-thickness retinal necrosis during the pathogenesis of MAIDS-related MCMV retinitis. This observation may extend to the pathogenesis of AIDS-related HCMV retinitis and other AIDS-related opportunistic virus infections.
Keywords: AIDS; Cell death pathway; Human cytomegalovirus; MAIDS; Murine cytomegalovirus; Pyroptosis; Retinitis.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no competing financial interests.
Figures
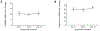

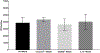
References
-
- Alston Carter JJ, Dix RD, I. C, 2017. Cytomegalovirus and the-Eye: AIDS-Related Retinitis and Beyond. Herpesviridae 42.
-
- Alston CI, Dix RD, 2017. Reduced frequency of murine cytomegalovirus retinitis in C57BL/6 mice correlates with low levels of suppressor of cytokine signaling (SOCS)1 and SOCS3 expression within the eye during corticosteroid-induced immunosuppression. Cytokine 97, 38–41. 10.1016/j.cyto.2017.05.021 - DOI - PMC - PubMed
-
- Atherton SS, Newell CK, Kanter MY, Cousins SW, 1991. Retinitis in euthymic mice following inoculation of murine cytomegalovirus (MCMV) via the supraciliary route. Curr Eye Res 10, 667–677. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

