Web-based, rapid and contactless management of ambulatory patients for SARS-CoV-2-testing
- PMID: 34098882
- PMCID: PMC8182346
- DOI: 10.1186/s12879-021-06249-7
Web-based, rapid and contactless management of ambulatory patients for SARS-CoV-2-testing
Abstract
Background: During the SARS-CoV-2 pandemic a mass casualty incident of ambulatory patients occurred at the COVID-19 rapid response infrastructure (CRRI) facility at the University Hospital of Cologne (UHC). We report the development of a patient-centred mobile-device solution to support efficient management of the facility, triage of patients and rapid delivery of test results.
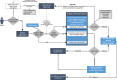
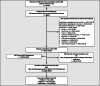
Methods: The UHC-Corona Web Tool (CWT) was developed as a web-based software useable on each patient's smartphone. It provides, among others, a self-reported medical history including type and duration of symptoms and potential risk contacts and links all retrieved information to the digital patient chart via a QR code. It provides scheduling of outpatient appointments and automated transmission of SARS-CoV-2 test results.
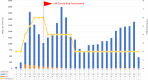
Results: The UHC-CWT was launched on 9 April 2020. It was used by 28,652 patients until 31 August 2020. Of those, 15,245 (53,2%) consulted the CRRI, representing 43,1% of all CRRI patients during the observed period. There were 8304 (29,0%) specifications concerning travel history and 17,145 (59,8%) indications of ≥1 symptom of SARS-CoV-2 infection. The most frequently indicated symptoms were sore throat (60,0%), headache (50,7%), common cold (45,1%) and cough (42,6%) while 11,057 (40,2%) patients did not report any symptoms. After implementation of the UHC-CWT, the amount of patient contacts per physician rose from 38 to 98,7 per day. The personnel for communication of test results were reduced from four on seven days to one on five days.
Conclusion: The UHC-CWT is an effective digital solution for management of large numbers of outpatients for SARS-CoV-2 testing.
Keywords: COVID-19 pandemic; Digital medicine; SARS-CoV-2 testing strategy; Web-based patient management.
Conflict of interest statement
JS reports research grants from Basilea Pharmaceuticals Inc., and travel grants from Meta-Alexander Foundation and from German Society for Infectious Diseases e.V. outside the submitted work; OAC is supported by the German Federal Ministry of Research and Education, is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy - CECAD, EXC 2030–390661388 and has received research grants from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen, Medicines Company, Melinta, Merck/MSD, Octapharma, Pfizer, Scynexis, is a consultant to Actelion, Allecra, Amplyx, Astellas, Basilea, Biosys, Cidara, Da Volterra, Entasis, F2G, Gilead, Matinas, MedPace, Menarini, Merck/MSD, Mylan, Nabriva, Noxxon, Octapharma, Paratek, Pfizer, PSI, Roche Diagnostics, Scynexis, and Shionogi, and received lecture honoraria from Al-Jazeera Pharmaceuticals, Astellas, Basilea, Gilead, Grupo Biotoscana, Merck/MSD and Pfizer outside the submitted work; TN-S declares no conflict of interest; CF is an employee of Healex GmbH and otherwise declares no conflict of interest; SS declares no conflict of interest; LP declares no conflict of interest; BB declares no conflict of interest; CL declares no conflict of interest; GL declares no conflict of interest.
Figures
References
-
- Augustin M, Schommers P, Suárez I, Koehler P, Gruell H, Klein F, Maurer C, Langerbeins P, Priesner V, Schmidt-Hellerau K, Malin JJ, Stecher M, Jung N, Wiesmüller G, Meissner A, Zweigner J, Langebartels G, Kolibay F, Suárez V, Burst V, Valentin P, Schedler D, Cornely OA, Hallek M, Fätkenheuer G, Rybniker J, Lehmann C. Rapid response infrastructure for pandemic preparedness in a tertiary care hospital: lessons learned from the COVID-19 outbreak in Cologne, Germany, February to March 2020. Euro Surveill. 2020;25(21):2000531. 10.2807/1560-7917.ES.2020.25.21.2000531. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

