Mepolizumab improves clinical outcomes in patients with severe asthma and comorbid conditions
- PMID: 34098955
- PMCID: PMC8182929
- DOI: 10.1186/s12931-021-01746-4
Mepolizumab improves clinical outcomes in patients with severe asthma and comorbid conditions
Abstract
Background: Comorbidities can complicate the management of severe asthma; therefore, the presence of comorbid conditions or traits often need to be considered when considering treatment options for patients with severe asthma. The aim of this analysis is to investigate the efficacy of mepolizumab in patients with severe eosinophilic asthma and comorbidities.
Methods: This was a post hoc analysis (GSK ID:209140) of data from the Phase IIb/III studies DREAM, MENSA, SIRIUS, and MUSCA. Patients aged ≥ 12 years with severe eosinophilic asthma were randomized to: mepolizumab 750, 250, or 75 mg intravenously or placebo (DREAM); mepolizumab 75 mg intravenously or 100 mg subcutaneously or placebo (MENSA); or mepolizumab 100 mg subcutaneously or placebo (SIRIUS and MUSCA) every 4 weeks for 24 weeks in SIRIUS and MUSCA, 32 weeks in MENSA or 52 weeks in DREAM. In this analysis the primary endpoint was the annual rate of clinically significant exacerbations; secondary endpoints were Asthma Control Questionnaire-5 score, St George's Respiratory Questionnaire total score, and pre-bronchodilator forced expiratory volume in 1 s at study end. Subgroups were based on comorbidities at baseline.
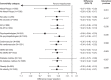
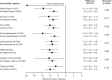
Results: Overall, 1878 patients received placebo (n = 689) or mepolizumab (n = 1189). Across all comorbidity subgroups mepolizumab reduced the rate of clinically significant exacerbations by 44-68% versus placebo, improved Asthma Control Questionnaire-5 score by 0.27-0.59 points, and improved St George's Respiratory Questionnaire total score by 5.0-11.6 points. Pre-bronchodilator forced expiratory volume in 1 s was improved by 27.1-286.9 mL in all but one comorbidity subgroup, the diabetes mellitus subgroup.
Conclusions: Mepolizumab reduces exacerbations, and improves asthma control, health-related quality of life, and lung function in patients with severe eosinophilic asthma despite comorbid conditions, including upper respiratory conditions, psychopathologies, cardiovascular conditions, gastroesophageal reflux disease, diabetes mellitus, and obesity.
Trial registration: https://clinicaltrials.gov/ DREAM, MEA112997/NCT01000506; MENSA, MEA115588/NCT01691521; SIRIUS, MEA115575/NCT01842607; MUSCA, 200862/NCT02281318.
Keywords: Cardiovascular; Comorbidities; Mepolizumab; Severe eosinophilic asthma; Treatable traits; Upper respiratory.
Conflict of interest statement
PGG reports grants and personal fees from GSK; grants and personal fees from AstraZeneca; and personal fees from Novartis and Sanofi. CMP, ESB, SAM, SGS and SWY are employees of GSK and hold stocks/shares. MF is a former employee of GSK and holds stocks/shares, and is currently employed by Vertex Pharmaceuticals. GLC has acted as a consultant, for AstraZeneca, Genentech, Boehringer Ingelheim, and Teva; attended a speakers' bureau with AstraZeneca, Genentech, and Circassia; and received research grants from AstraZeneca and institutional grants from AstraZeneca, Genentech, Boehringer Ingelheim, and GSK. EHB reports grants from AstraZeneca, GSK, and Novartis; and personal fees from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Sanofi/Regeneron, Teva, Sterna, and Vectura.
Figures


Similar articles
-
Impact of baseline clinical asthma characteristics on the response to mepolizumab: a post hoc meta-analysis of two Phase III trials.Respir Res. 2021 Jun 22;22(1):184. doi: 10.1186/s12931-021-01767-z. Respir Res. 2021. PMID: 34158028 Free PMC article. Review.
-
Mepolizumab reduces exacerbations in patients with severe eosinophilic asthma, irrespective of body weight/body mass index: meta-analysis of MENSA and MUSCA.Respir Res. 2019 Jul 30;20(1):169. doi: 10.1186/s12931-019-1134-7. Respir Res. 2019. PMID: 31362741 Free PMC article. Clinical Trial.
-
Effect of mepolizumab in severe eosinophilic asthma according to omalizumab eligibility.Respir Med. 2019 Jul-Aug;154:69-75. doi: 10.1016/j.rmed.2019.06.004. Epub 2019 Jun 8. Respir Med. 2019. PMID: 31220806
-
Efficacy and safety of mepolizumab in Korean patients with severe eosinophilic asthma from the DREAM and MENSA studies.Korean J Intern Med. 2021 Mar;36(2):362-370. doi: 10.3904/kjim.2019.198. Epub 2020 May 26. Korean J Intern Med. 2021. PMID: 32450626 Free PMC article.
-
Mepolizumab for Treating Severe Eosinophilic Asthma: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Feb;36(2):131-144. doi: 10.1007/s40273-017-0571-8. Pharmacoeconomics. 2018. PMID: 28933002 Review.
Cited by
-
Association of Obesity and Severe Asthma in Adults.J Clin Med. 2024 Jun 14;13(12):3474. doi: 10.3390/jcm13123474. J Clin Med. 2024. PMID: 38930006 Free PMC article. Review.
-
Challenges in severe asthma: Do we need new drugs or new biomarkers?Front Med (Lausanne). 2022 Sep 27;9:921967. doi: 10.3389/fmed.2022.921967. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36237537 Free PMC article. Review.
-
The effect of mepolizumab dosage form on treatment outcomes in severe asthma.Front Med (Lausanne). 2025 Apr 17;12:1537074. doi: 10.3389/fmed.2025.1537074. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40330778 Free PMC article.
-
Anti-IL-5 therapies for asthma.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD010834. doi: 10.1002/14651858.CD010834.pub4. Cochrane Database Syst Rev. 2022. PMID: 35838542 Free PMC article.
-
Lung function trajectories in a cohort of patients with moderate-to-severe asthma on mepolizumab, omalizumab, or dupilumab.Allergy. 2024 May;79(5):1195-1207. doi: 10.1111/all.16002. Epub 2024 Jan 2. Allergy. 2024. PMID: 38164813 Free PMC article.
References
-
- Global Asthma Network. The Global Asthma Report. 2018. http://www.globalasthmareport.org/Global%20Asthma%20Report%202018.pdf. Accessed 30 Sept 2019.
-
- Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–346. doi: 10.1136/thoraxjnl-2015-207630. - DOI - PubMed

