Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma
- PMID: 34099013
- PMCID: PMC8183071
- DOI: 10.1186/s13046-021-01961-3
Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma
Abstract
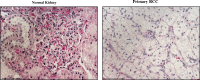
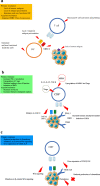
Vascular endothelial growth factor tyrosine kinase inhibitors (VEGF-TKIs) have been the mainstay of treatment for patients with advanced renal cell carcinoma (RCC). Despite its early promising results in decreasing or delaying the progression of RCC in patients, VEGF-TKIs have provided modest benefits in terms of disease-free progression, as 70% of the patients who initially respond to the treatment later develop drug resistance, with 30% of the patients innately resistant to VEGF-TKIs. In the past decade, several molecular and genetic mechanisms of VEGF-TKI resistance have been reported. One of the mechanisms of VEGF-TKIs is inhibition of the classical angiogenesis pathway. However, recent studies have shown the restoration of an alternative angiogenesis pathway in modulating resistance. Further, in the last 5 years, immune checkpoint inhibitors (ICIs) have revolutionized RCC treatment. Although some patients exhibit potent responses, a non-negligible number of patients are innately resistant or develop resistance within a few months to ICI therapy. Hence, an understanding of the mechanisms of VEGF-TKI and ICI resistance will help in formulating useful knowledge about developing effective treatment strategies for patients with advanced RCC. In this article, we review recent findings on the emerging understanding of RCC pathology, VEGF-TKI and ICI resistance mechanisms, and potential avenues to overcome these resistance mechanisms through rationally designed combination therapies.
Keywords: Clear cell renal carcinoma; Epithelial mesenchymal transition; Hypoxia; Metastatic renal cell carcinoma; Sunitinib; Vascular endothelial growth factor, tyrosine kinase inhibitor.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

