A novel splicing variant of ANXA11 in a patient with amyotrophic lateral sclerosis: histologic and biochemical features
- PMID: 34099057
- PMCID: PMC8186038
- DOI: 10.1186/s40478-021-01202-w
A novel splicing variant of ANXA11 in a patient with amyotrophic lateral sclerosis: histologic and biochemical features
Erratum in
-
Correction to: A novel splicing variant of ANXA11 in a patient with amyotrophic lateral sclerosis: histologic and biochemical features.Acta Neuropathol Commun. 2021 Jun 28;9(1):115. doi: 10.1186/s40478-021-01217-3. Acta Neuropathol Commun. 2021. PMID: 34183068 Free PMC article. No abstract available.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
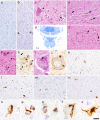
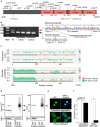
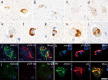
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

