Probing ion channel macromolecular interactions using fluorescence resonance energy transfer
- PMID: 34099178
- PMCID: PMC9009374
- DOI: 10.1016/bs.mie.2021.01.047
Probing ion channel macromolecular interactions using fluorescence resonance energy transfer
Abstract
Ion channels are macromolecular complexes whose functions are exquisitely tuned by interacting proteins. Fluorescence resonance energy transfer (FRET) is a powerful methodology that is adept at quantifying ion channel protein-protein interactions in living cells. For FRET experiments, the interacting partners are tagged with appropriate donor and acceptor fluorescent proteins. If the fluorescently-labeled molecules are in close proximity, then photoexcitation of the donor results in non-radiative energy transfer to the acceptor, and subsequent fluorescence emission of the acceptor. The stoichiometry of ion channel interactions and their relative binding affinities can be deduced by quantifying both the FRET efficiency and the total number of donors and acceptors in a given cell. In this chapter, we discuss general considerations for FRET analysis of biological interactions, various strategies for estimating FRET efficiencies, and detailed protocols for construction of binding curves and determination of stoichiometry. We focus on implementation of FRET assays using a flow cytometer given its amenability for high-throughput data acquisition, enhanced accessibility, and robust analysis. This versatile methodology permits mechanistic dissection of dynamic changes in ion channel interactions.
Keywords: FRET 2-hybrid assay; Flow cytometry; Fluorescence resonance energy transfer; Live-cell binding; Stoichiometry.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
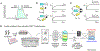

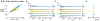

Similar articles
-
Fluorescence resonance energy transfer-based stoichiometry in living cells.Biophys J. 2002 Dec;83(6):3652-64. doi: 10.1016/S0006-3495(02)75365-4. Biophys J. 2002. PMID: 12496132 Free PMC article.
-
Flow cytometric measurement of fluorescence (Förster) resonance energy transfer from cyan fluorescent protein to yellow fluorescent protein using single-laser excitation at 458 nm.Cytometry A. 2003 May;53(1):39-54. doi: 10.1002/cyto.a.10037. Cytometry A. 2003. PMID: 12701131
-
Anomalous surplus energy transfer observed with multiple FRET acceptors.PLoS One. 2009 Nov 25;4(11):e8031. doi: 10.1371/journal.pone.0008031. PLoS One. 2009. PMID: 19946626 Free PMC article.
-
Optical methods in the study of protein-protein interactions.Adv Exp Med Biol. 2010;674:33-42. doi: 10.1007/978-1-4419-6066-5_4. Adv Exp Med Biol. 2010. PMID: 20549938 Review.
-
Quantitative intensity-based FRET approaches--a comparative snapshot.Biophys J. 2012 Nov 7;103(9):1821-7. doi: 10.1016/j.bpj.2012.09.031. Biophys J. 2012. PMID: 23199910 Free PMC article. Review.
Cited by
-
Bestrophin-2 and glutamine synthetase form a complex for glutamate release.Nature. 2022 Nov;611(7934):180-187. doi: 10.1038/s41586-022-05373-x. Epub 2022 Oct 26. Nature. 2022. PMID: 36289327 Free PMC article.
-
Arrhythmia-associated calmodulin variants interact with KCNQ1 to confer aberrant membrane trafficking and function.PNAS Nexus. 2023 Oct 14;2(11):pgad335. doi: 10.1093/pnasnexus/pgad335. eCollection 2023 Nov. PNAS Nexus. 2023. PMID: 37965565 Free PMC article.
-
Elementary mechanisms of calmodulin regulation of NaV1.5 producing divergent arrhythmogenic phenotypes.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2025085118. doi: 10.1073/pnas.2025085118. Proc Natl Acad Sci U S A. 2021. PMID: 34021086 Free PMC article.
-
On the pixel selection criterion for the calculation of the Pearson's correlation coefficient in fluorescence microscopy.J Microsc. 2025 Mar;297(3):304-315. doi: 10.1111/jmi.13273. Epub 2024 Feb 13. J Microsc. 2025. PMID: 38349020 Free PMC article.
-
Selective posttranslational inhibition of CaVβ1-associated voltage-dependent calcium channels with a functionalized nanobody.Nat Commun. 2022 Dec 9;13(1):7556. doi: 10.1038/s41467-022-35025-7. Nat Commun. 2022. PMID: 36494348 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

