Production and purification of TRPV2 and TRPV5 for structural and functional studies
- PMID: 34099181
- PMCID: PMC8610384
- DOI: 10.1016/bs.mie.2021.02.007
Production and purification of TRPV2 and TRPV5 for structural and functional studies
Abstract
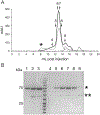
The transient receptor potential (TRP) vanilloid 2 (TRPV2) and TRP vanilloid 5 (TRPV5) cation channels play an important role in various physiological and pathophysiological processes. The heterologous expression and purification of these channels is critical for functional and structural characterization of these important proteins. Full-length rat TRPV2 and rabbit TRPV5 can both be expressed in Saccharomyces cerevisiae and affinity purified using the 1D4 epitope and antibody to yield pure, functional channels. Further, these channels can be reconstituted into lipid nanodiscs for a more functionally relevant environment. Presented here are protocols for the expression of full-length rat TRPV2 and rabbit TRPV5 in Saccharomyces cerevisiae, their affinity purification, and their reconstitution into nanodiscs for structural and functional studies.
Keywords: 1D4 antibody; Affinity protein purification; Nanodiscs; Saccharomyces cerevisiae protein expression; TRPV2; TRPV5; Transient receptor potential channel.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
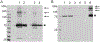
Similar articles
-
Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails.Amino Acids. 2011 Feb;40(2):741-8. doi: 10.1007/s00726-010-0712-2. Epub 2010 Aug 5. Amino Acids. 2011. PMID: 20686800
-
Molecular mechanism of TRPV2 channel modulation by cannabidiol.Elife. 2019 Sep 30;8:e48792. doi: 10.7554/eLife.48792. Elife. 2019. PMID: 31566564 Free PMC article.
-
Structural insight into TRPV5 channel function and modulation.Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):8869-8878. doi: 10.1073/pnas.1820323116. Epub 2019 Apr 11. Proc Natl Acad Sci U S A. 2019. PMID: 30975749 Free PMC article.
-
Trafficking of Stretch-Regulated TRPV2 and TRPV4 Channels Inferred Through Interactomics.Biomolecules. 2019 Nov 27;9(12):791. doi: 10.3390/biom9120791. Biomolecules. 2019. PMID: 31783610 Free PMC article. Review.
-
Vanilloid transient receptor potential cation channels: an overview.Curr Pharm Des. 2008;14(1):18-31. doi: 10.2174/138161208783330763. Curr Pharm Des. 2008. PMID: 18220815 Review.
Cited by
-
Structural basis of the activation of TRPV5 channels by long-chain acyl-Coenzyme-A.Nat Commun. 2023 Sep 21;14(1):5883. doi: 10.1038/s41467-023-41577-z. Nat Commun. 2023. PMID: 37735536 Free PMC article.
-
Structural basis of TRPV5 regulation by physiological and pathophysiological modulators.Cell Rep. 2022 Apr 26;39(4):110737. doi: 10.1016/j.celrep.2022.110737. Cell Rep. 2022. PMID: 35476976 Free PMC article.
-
Functional and structural insights into activation of TRPV2 by weak acids.EMBO J. 2024 Jun;43(11):2264-2290. doi: 10.1038/s44318-024-00106-4. Epub 2024 Apr 26. EMBO J. 2024. PMID: 38671253 Free PMC article.
-
Molecular details of ruthenium red pore block in TRPV channels.EMBO Rep. 2024 Feb;25(2):506-523. doi: 10.1038/s44319-023-00050-0. Epub 2024 Jan 15. EMBO Rep. 2024. PMID: 38225355 Free PMC article.
References
-
- Bayburt TH, Grinkova YV, & Sligar SG (2002). Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Letters, 2(8), 853–856.
-
- Denisov IG, et al. (2004). Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. Journal of the American Chemical Society, 126(11), 3477–3487. - PubMed
-
- Figler RA, et al. (2000). Use of chemical chaperones in the yeast Saccharomyces cerevisiae to enhance heterologous membrane protein expression: High-yield expression and purification of human P-glycoprotein. Archives of Biochemistry and Biophysics, 376(1), 34–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

