Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders
- PMID: 34099189
- PMCID: PMC8458480
- DOI: 10.1016/j.biopsych.2021.02.972
Sex-Dependent Shared and Nonshared Genetic Architecture Across Mood and Psychotic Disorders
Abstract
Background: Sex differences in incidence and/or presentation of schizophrenia (SCZ), major depressive disorder (MDD), and bipolar disorder (BIP) are pervasive. Previous evidence for shared genetic risk and sex differences in brain abnormalities across disorders suggest possible shared sex-dependent genetic risk.
Methods: We conducted the largest to date genome-wide genotype-by-sex (G×S) interaction of risk for these disorders using 85,735 cases (33,403 SCZ, 19,924 BIP, and 32,408 MDD) and 109,946 controls from the PGC (Psychiatric Genomics Consortium) and iPSYCH.
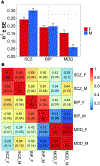
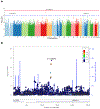
Results: Across disorders, genome-wide significant single nucleotide polymorphism-by-sex interaction was detected for a locus encompassing NKAIN2 (rs117780815, p = 3.2 × 10-8), which interacts with sodium/potassium-transporting ATPase (adenosine triphosphatase) enzymes, implicating neuronal excitability. Three additional loci showed evidence (p < 1 × 10-6) for cross-disorder G×S interaction (rs7302529, p = 1.6 × 10-7; rs73033497, p = 8.8 × 10-7; rs7914279, p = 6.4 × 10-7), implicating various functions. Gene-based analyses identified G×S interaction across disorders (p = 8.97 × 10-7) with transcriptional inhibitor SLTM. Most significant in SCZ was a MOCOS gene locus (rs11665282, p = 1.5 × 10-7), implicating vascular endothelial cells. Secondary analysis of the PGC-SCZ dataset detected an interaction (rs13265509, p = 1.1 × 10-7) in a locus containing IDO2, a kynurenine pathway enzyme with immunoregulatory functions implicated in SCZ, BIP, and MDD. Pathway enrichment analysis detected significant G×S interaction of genes regulating vascular endothelial growth factor receptor signaling in MDD (false discovery rate-corrected p < .05).
Conclusions: In the largest genome-wide G×S analysis of mood and psychotic disorders to date, there was substantial genetic overlap between the sexes. However, significant sex-dependent effects were enriched for genes related to neuronal development and immune and vascular functions across and within SCZ, BIP, and MDD at the variant, gene, and pathway levels.
Keywords: Bipolar disorder; Genome-wide association study; Genotype-by-sex interaction; Major depressive disorder; Schizophrenia; Sex differences.
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures
JG is on the scientific advisory board for and has equity in Cala Health, a neuromodulation company, although this is unrelated to the topic in this study; and TLP is an employee of Concert Pharmaceuticals, also unrelated to this study. JWS is an unpaid member of the Bipolar/Depression Research Community Advisory Panel of 23andMe. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures
References
-
- Diflorio A, Jones I (2010): Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 22:437–452. - PubMed
-
- Erol A, Winham SJ, McElroy SL, Frye MA, Prieto ML, Cuellar-Barboza AB, et al. (2015): Sex differences in the risk of rapid cycling and other indicators of adverse illness course in patients with bipolar I and II disorder. Bipolar Disord. 17:670–676. - PubMed
-
- Falkenburg J, Tracy DK (2014): Sex and schizophrenia: A review of gender differences. Psychosis. 6:61–69.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

