Stable expression of the human thrombomodulin transgene in pig endothelial cells is associated with a reduction in the inflammatory response
- PMID: 34099346
- PMCID: PMC8511266
- DOI: 10.1016/j.cyto.2021.155580
Stable expression of the human thrombomodulin transgene in pig endothelial cells is associated with a reduction in the inflammatory response
Abstract
Background: Xenotransplantation is associated with an inflammatory response. The proinflammatory cytokine, TNF-α, downregulates the expression of thrombomodulin (TBM), and induces coagulation dysfunction. Although human (h) TBM-transgenic pigs (p) have been developed to reduce coagulation dysfunction, the effect of TNF-α on the expression of hTBM and its functional activity has not been fully investigated. The aims of this study were to investigate (i) whether the expression of hTBM on pig (p) cells is down-regulated during TNF-α stimulation, and (ii) whether cells from hTBM pigs regulate the inflammatory response.
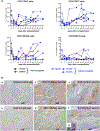
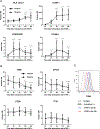
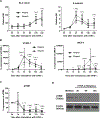
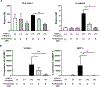
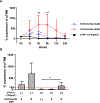
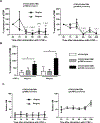
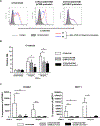
Methods: TNF-α-producing T, B, and natural killer cells in blood from baboons with pig heart or kidney xenografts were investigated by flow cytometry. TNF-α staining in the grafts was detected by immunohistochemistry. Aortic endothelial cells (AECs) from GTKO/CD46 and GTKO/CD46/hTBM pigs were stimulated by hTNF-α, and the expression of the inflammatory/coagulation regulatory protein, TBM, was investigated.
Results: After pig organ xenotransplantation, there was a trend to increases in TNF-α-producing T and natural killer cells in the blood of baboons. In vitro observations demonstrated that after hTNF-α stimulation, there was a significant reduction in the expression of endogenous pTBM on pAECs, and a significant increase in the expression of inflammatory molecules. Blocking of NF-κB signaling significantly up-regulated pTBM expression, and suppressed the inflammatory response induced by hTNF-α in pAECs. Whereas the expression of pTBM mRNA was significantly reduced by hTNF-α stimulation, hTBM expression on the GTKO/CD46/hTBM pAECs was not affected. Furthermore, after hTNF-α stimulation, there was significant suppression of expression of inflammatory molecules on GTKO/CD46/hTBM pAECs compared to GTKO/CD46 pAECs.
Conclusions: The stable expression of hTBM in pig cells may locally regulate the inflammatory response. This will help suppress the inflammatory response and prevent coagulation dysregulation after xenotransplantation.
Keywords: Inflammation; Pig, genetically-engineered; Thrombomodulin; Xenotransplantation.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure of conflict of interest
Kasinath Kuravi, Will Eyestone, Carol Phelps and David Ayares are employees of Revivicor, Blacksburg, VA. The other authors declare no conflicts of interest.
Figures

Similar articles
-
Human thrombomodulin regulates complement activation as well as the coagulation cascade in xeno-immune response.Xenotransplantation. 2015 Jul-Aug;22(4):260-72. doi: 10.1111/xen.12173. Epub 2015 Jul 14. Xenotransplantation. 2015. PMID: 26179123
-
Transgenic Expression of Human Thrombomodulin Inhibits HMGB1-Induced Porcine Aortic Endothelial Cell Activation.Transplantation. 2016 Sep;100(9):1871-9. doi: 10.1097/TP.0000000000001188. Transplantation. 2016. PMID: 27077599
-
Expression of human thrombomodulin on porcine endothelial cells can reduce platelet aggregation but did not reduce activation of complement or endothelium - an experimental study.Transpl Int. 2020 Apr;33(4):437-449. doi: 10.1111/tri.13573. Epub 2020 Feb 9. Transpl Int. 2020. PMID: 31926034
-
Overcoming Coagulation Dysregulation in Pig Solid Organ Transplantation in Nonhuman Primates: Recent Progress.Transplantation. 2018 Jul;102(7):1050-1058. doi: 10.1097/TP.0000000000002171. Transplantation. 2018. PMID: 29538262 Free PMC article. Review.
-
Justification of specific genetic modifications in pigs for clinical organ xenotransplantation.Xenotransplantation. 2019 Jul;26(4):e12516. doi: 10.1111/xen.12516. Epub 2019 Apr 15. Xenotransplantation. 2019. PMID: 30989742 Free PMC article. Review.
Cited by
-
Characterizing coagulation responses in humans and nonhuman primates following kidney xenotransplantation-A narrative review.Am J Hematol. 2025 Feb;100(2):285-295. doi: 10.1002/ajh.27506. Epub 2024 Oct 15. Am J Hematol. 2025. PMID: 39404060 Free PMC article. Review.
-
Developments in kidney xenotransplantation.Front Immunol. 2024 Jan 11;14:1242478. doi: 10.3389/fimmu.2023.1242478. eCollection 2023. Front Immunol. 2024. PMID: 38274798 Free PMC article. Review.
-
Multipotent Mesenchymal Stromal Cells Interact and Support Islet of Langerhans Viability and Function.Front Endocrinol (Lausanne). 2022 Feb 9;13:822191. doi: 10.3389/fendo.2022.822191. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35222280 Free PMC article. Review.
-
The Role of Interleukin-6 (IL-6) in the Systemic Inflammatory Response in Xenograft Recipients and in Pig Kidney Xenograft Failure.Front Immunol. 2021 Dec 8;12:788949. doi: 10.3389/fimmu.2021.788949. eCollection 2021. Front Immunol. 2021. PMID: 34956220 Free PMC article.
References
-
- Langin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564: 430–433. - PubMed
-
- Cowan PJ. Coagulation and the xenograft endothelium. Xenotransplantation. 2007;14: 7–12. - PubMed
-
- Cooper DK, Ezzelarab MB, Hara H, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23: 83–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

