Viewing Ambiguous Social Interactions Increases Functional Connectivity between Frontal and Temporal Nodes of the Social Brain
- PMID: 34099508
- PMCID: PMC8276745
- DOI: 10.1523/JNEUROSCI.0870-20.2021
Viewing Ambiguous Social Interactions Increases Functional Connectivity between Frontal and Temporal Nodes of the Social Brain
Abstract
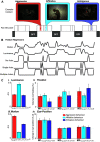
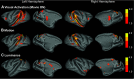
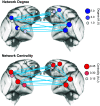
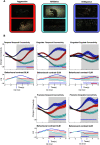
Social behavior is coordinated by a network of brain regions, including those involved in the perception of social stimuli and those involved in complex functions, such as inferring perceptual and mental states and controlling social interactions. The properties and function of many of these regions in isolation are relatively well understood, but less is known about how these regions interact while processing dynamic social interactions. To investigate whether the functional connectivity between brain regions is modulated by social context, we collected fMRI data from male monkeys (Macaca mulatta) viewing videos of social interactions labeled as "affiliative," "aggressive," or "ambiguous." We show activation related to the perception of social interactions along both banks of the superior temporal sulcus, parietal cortex, medial and lateral frontal cortex, and the caudate nucleus. Within this network, we show that fronto-temporal functional connectivity is significantly modulated by social context. Crucially, we link the observation of specific behaviors to changes in functional connectivity within our network. Viewing aggressive behavior was associated with a limited increase in temporo-temporal and a weak increase in cingulate-temporal connectivity. By contrast, viewing interactions where the outcome was uncertain was associated with a pronounced increase in temporo-temporal, and cingulate-temporal functional connectivity. We hypothesize that this widespread network synchronization occurs when cingulate and temporal areas coordinate their activity when more difficult social inferences are being made.SIGNIFICANCE STATEMENT Processing social information from our environment requires the activation of several brain regions, which are concentrated within the frontal and temporal lobes. However, little is known about how these areas interact to facilitate the processing of different social interactions. Here we show that functional connectivity within and between the frontal and temporal lobes is modulated by social context. Specifically, we demonstrate that viewing social interactions where the outcome was unclear is associated with increased synchrony within and between the cingulate cortex and temporal cortices. These findings suggest that the coordination between the cingulate and temporal cortices is enhanced when more difficult social inferences are being made.
Keywords: fMRI; face-processing; monkey; social cognition.
Copyright © 2021 Ainsworth et al.
Figures



References
-
- Ainsworth M, Browncross H, Mitchell DJ, Mitchell AS, Passingham RE, Buckley MJ, Duncan J, Bell AH (2018) Functional reorganisation and recovery following cortical lesions: a preliminary study in macaque monkeys. Neuropsychologia 119:382–391. 10.1016/j.neuropsychologia.2018.08.024 - DOI - PMC - PubMed
-
- Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, de Schotten T (2019) Subcortical anatomy of the default mode network: a functional and structural connectivity study. bioRxiv 528679.
Grants and funding
LinkOut - more resources
Full Text Sources
