Multicenter Trial of a Tubeless, On-Body Automated Insulin Delivery System With Customizable Glycemic Targets in Pediatric and Adult Participants With Type 1 Diabetes
- PMID: 34099518
- PMCID: PMC8323171
- DOI: 10.2337/dc21-0172
Multicenter Trial of a Tubeless, On-Body Automated Insulin Delivery System With Customizable Glycemic Targets in Pediatric and Adult Participants With Type 1 Diabetes
Abstract
Objective: Advances in diabetes technology have transformed the treatment paradigm for type 1 diabetes, yet the burden of disease is significant. We report on a pivotal safety study of the first tubeless, on-body automated insulin delivery system with customizable glycemic targets.
Research design and methods: This single-arm, multicenter, prospective study enrolled 112 children (age 6-13.9 years) and 129 adults (age 14-70 years). A 2-week standard therapy phase (usual insulin regimen) was followed by 3 months of automated insulin delivery. Primary safety outcomes were incidence of severe hypoglycemia and diabetic ketoacidosis. Primary effectiveness outcomes were change in HbA1c and percent time in sensor glucose range 70-180 mg/dL ("time in range").
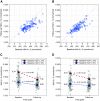
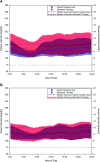
Results: A total of 235 participants (98% of enrolled, including 111 children and 124 adults) completed the study. HbA1c was significantly reduced in children by 0.71% (7.8 mmol/mol) (mean ± SD: 7.67 ± 0.95% to 6.99 ± 0.63% [60 ± 10.4 mmol/mol to 53 ± 6.9 mmol/mol], P < 0.0001) and in adults by 0.38% (4.2 mmol/mol) (7.16 ± 0.86% to 6.78 ± 0.68% [55 ± 9.4 mmol/mol to 51 ± 7.4 mmol/mol], P < 0.0001). Time in range was improved from standard therapy by 15.6 ± 11.5% or 3.7 h/day in children and 9.3 ± 11.8% or 2.2 h/day in adults (both P < 0.0001). This was accomplished with a reduction in time in hypoglycemia <70 mg/dL among adults (median [interquartile range]: 2.00% [0.63, 4.06] to 1.09% [0.46, 1.75], P < 0.0001), while this parameter remained the same in children. There were three severe hypoglycemia events not attributable to automated insulin delivery malfunction and one diabetic ketoacidosis event from an infusion site failure.
Conclusions: This tubeless automated insulin delivery system was safe and allowed participants to significantly improve HbA1c levels and time in target glucose range with a very low occurrence of hypoglycemia.
Trial registration: ClinicalTrials.gov NCT04196140.
© 2021 by the American Diabetes Association.
Figures
References
-
- American Diabetes Association . 6. Glycemic Targets: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl. 1):S66–S76 - PubMed
-
- Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 - PubMed
-
- Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–2227 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

