Accurate genomic variant detection in single cells with primary template-directed amplification
- PMID: 34099548
- PMCID: PMC8214697
- DOI: 10.1073/pnas.2024176118
Accurate genomic variant detection in single cells with primary template-directed amplification
Abstract
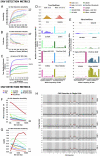
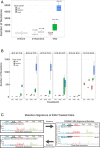
Improvements in whole genome amplification (WGA) would enable new types of basic and applied biomedical research, including studies of intratissue genetic diversity that require more accurate single-cell genotyping. Here, we present primary template-directed amplification (PTA), an isothermal WGA method that reproducibly captures >95% of the genomes of single cells in a more uniform and accurate manner than existing approaches, resulting in significantly improved variant calling sensitivity and precision. To illustrate the types of studies that are enabled by PTA, we developed direct measurement of environmental mutagenicity (DMEM), a tool for mapping genome-wide interactions of mutagens with single living human cells at base-pair resolution. In addition, we utilized PTA for genome-wide off-target indel and structural variant detection in cells that had undergone CRISPR-mediated genome editing, establishing the feasibility for performing single-cell evaluations of biopsies from edited tissues. The improved precision and accuracy of variant detection with PTA overcomes the current limitations of accurate WGA, which is the major obstacle to studying genetic diversity and evolution at cellular resolution.
Keywords: genome editing off-target; mutagenesis; single-cell sequencing; whole genome amplification.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: C.G. is a Co-Founder and Board Member of BioSkryb Genomics, which is commercializing primary template-directed amplification.
Figures




References
-
- Gawad C., Koh W., Quake S. R., Single-cell genome sequencing: Current state of the science. Nat. Rev. Genet. 17, 175–188 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

