A Newly Synthesized Flavone from Luteolin Escapes from COMT-Catalyzed Methylation and Inhibits Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages via JNK, p38 and NF-κB Signaling Pathways
- PMID: 34099595
- PMCID: PMC9628824
- DOI: 10.4014/jmb.2104.04027
A Newly Synthesized Flavone from Luteolin Escapes from COMT-Catalyzed Methylation and Inhibits Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages via JNK, p38 and NF-κB Signaling Pathways
Abstract
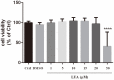
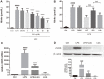
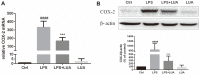
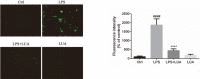
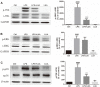
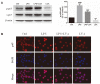
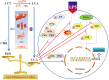
Luteolin is a common dietary flavone possessing potent anti-inflammatory activities. However, when administrated in vivo, luteolin becomes methylated by catechol-O-methyltransferases (COMT) owing to the catechol ring in the chemical structure, which largely diminishes its anti-inflammatory effect. In this study, we made a modification on luteolin, named LUA, which was generated by the chemical reaction between luteolin and 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH). Without a catechol ring in the chemical structure, this new flavone could escape from the COMT-catalyzed methylation, thus affording the potential to exert its functions in the original form when administrated in the organism. Moreover, an LPS-stimulated RAW cell model was applied to detect the anti-inflammatory properties. LUA showed much more superior inhibitory effect on LPS-induced production of NO than diosmetin (a major methylated form of luteolin) and significantly suppressed upregulation of iNOS and COX-2 in macrophages. LUA treatment dramatically reduced LPS-stimulated reactive oxygen species (ROS) and mRNA levels of pro-inflammatory mediators such as IL-1β, IL-6, IL-8 and IFN-β. Furthermore, LUA significantly reduced the phosphorylation of JNK and p38 without affecting that of ERK. LUA also inhibited the activation of NF-κB through suppression of p65 phosphorylation and nuclear translocation.
Keywords: 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH); Luteolin; catechol-O-methyltransferases (COMT); inflammation.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures
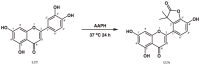

Similar articles
-
A newly synthesized flavone avoids COMT-catalyzed methylation and mitigates myocardial ischemia/reperfusion injury in H9C2 cells via JNK and P38 pathways.Iran J Basic Med Sci. 2024;27(4):492-499. doi: 10.22038/IJBMS.2023.74358.16149. Iran J Basic Med Sci. 2024. PMID: 38419895 Free PMC article.
-
Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation.Int J Mol Sci. 2020 Mar 16;21(6):2007. doi: 10.3390/ijms21062007. Int J Mol Sci. 2020. PMID: 32187984 Free PMC article.
-
Ethanol extract of Poria cocos reduces the production of inflammatory mediators by suppressing the NF-kappaB signaling pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages.BMC Complement Altern Med. 2014 Mar 15;14:101. doi: 10.1186/1472-6882-14-101. BMC Complement Altern Med. 2014. PMID: 24628870 Free PMC article.
-
Immunopharmacological Activities of Luteolin in Chronic Diseases.Int J Mol Sci. 2023 Jan 21;24(3):2136. doi: 10.3390/ijms24032136. Int J Mol Sci. 2023. PMID: 36768462 Free PMC article. Review.
-
From nature to therapy: Luteolin's potential as an immune system modulator in inflammatory disorders.J Biochem Mol Toxicol. 2023 Nov;37(11):e23482. doi: 10.1002/jbt.23482. Epub 2023 Aug 2. J Biochem Mol Toxicol. 2023. PMID: 37530602 Review.
Cited by
-
Downregulation of IL-8 and IL-10 by the Activation of Ca2+-Activated K+ Channel KCa3.1 in THP-1-Derived M2 Macrophages.Int J Mol Sci. 2022 Aug 3;23(15):8603. doi: 10.3390/ijms23158603. Int J Mol Sci. 2022. PMID: 35955737 Free PMC article.
-
The Anti-Inflammatory Mechanism of Flaxseed Linusorbs on Lipopolysaccharide-Induced RAW 264.7 Macrophages by Modulating TLR4/NF-κB/MAPK Pathway.Foods. 2023 Jun 16;12(12):2398. doi: 10.3390/foods12122398. Foods. 2023. PMID: 37372610 Free PMC article.
-
A newly synthesized flavone avoids COMT-catalyzed methylation and mitigates myocardial ischemia/reperfusion injury in H9C2 cells via JNK and P38 pathways.Iran J Basic Med Sci. 2024;27(4):492-499. doi: 10.22038/IJBMS.2023.74358.16149. Iran J Basic Med Sci. 2024. PMID: 38419895 Free PMC article.
-
Heat-Killed Latilactobacillus sakei CNSC001WB and Lactobacillus pentosus WB693 Have an Anti-inflammatory Effect on LPS-Stimulated RAW 264.7 Cells.Probiotics Antimicrob Proteins. 2024 Oct;16(5):1875-1885. doi: 10.1007/s12602-023-10139-6. Epub 2023 Aug 17. Probiotics Antimicrob Proteins. 2024. PMID: 37589784
-
Luteolin alleviates Herpes Simplex Keratitis by inhibiting inflammatory responses via suppressing the PTGS2/NF-κB signaling pathway.Am J Transl Res. 2025 May 15;17(5):3307-3321. doi: 10.62347/IQUZ8416. eCollection 2025. Am J Transl Res. 2025. PMID: 40535652 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

