Effects of short-chain fatty acids in inhibiting HDAC and activating p38 MAPK are critical for promoting B10 cell generation and function
- PMID: 34099635
- PMCID: PMC8184914
- DOI: 10.1038/s41419-021-03880-9
Effects of short-chain fatty acids in inhibiting HDAC and activating p38 MAPK are critical for promoting B10 cell generation and function
Abstract
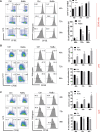
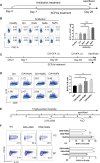
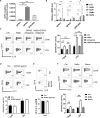
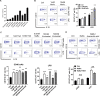
B10 cells are regulatory B cells capable of producing IL-10 for maintaining immune homeostasis. Dysregulation of B10 cells occurs in autoimmune and inflammatory diseases. Modulation or adoptive transfer of B10 cells is a promising therapeutic strategy. The short-chain fatty acids (SCFAs), the metabolites of microbiota, play a critical role in maintaining immune homeostasis and are the potential drugs for the modulation of B10 cells. It is not clear whether and how SCFAs upregulate the frequency of B10 cells. Here, we found that SCFAs could promote murine and human B10 cell generation in vitro. Upregulation of B10 cells by butyrate or pentanoate was also observed in either healthy mice, mice with dextran sodium sulfate (DSS)-induced colitis, or mice with collagen-induced arthritis. Moreover, SCFA treatment could ameliorate clinical scores of colitis and arthritis. Adoptive transfer of B cells pretreated with butyrate showed more alleviation of DSS-induced colitis than those without butyrate. A further study demonstrates that SCFAs upregulate B10 cells in a manner dependent on their histone deacetylase (HDAC) inhibitory activity and independent of the G-protein-coupled receptor pathway. Transcriptomic analysis indicated that the MAPK signaling pathway was enriched in B10 cells treated with butyrate. A study with inhibitors of ERK, JNK, and p38 MAPK demonstrated that activating p38 MAPK by butyrate is critical for the upregulation of B10 cells. Moreover, HDAC inhibitor has similar effects on B10 cells. Our study sheds light on the mechanism underlying B10 cell differentiation and function and provides a potential therapeutic strategy with SCFAs and HDAC inhibitors for inflammation and autoimmune diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells.J Anim Sci. 2018 Dec 3;96(12):5244-5252. doi: 10.1093/jas/sky373. J Anim Sci. 2018. PMID: 30252114 Free PMC article.
-
Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity.Nat Commun. 2020 Sep 8;11(1):4457. doi: 10.1038/s41467-020-18262-6. Nat Commun. 2020. PMID: 32901017 Free PMC article.
-
Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells.Biochem Biophys Res Commun. 2017 Apr 29;486(2):499-505. doi: 10.1016/j.bbrc.2017.03.071. Epub 2017 Mar 18. Biochem Biophys Res Commun. 2017. PMID: 28322790
-
Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides.Biomed Pharmacother. 2023 Jun;162:114586. doi: 10.1016/j.biopha.2023.114586. Epub 2023 Mar 28. Biomed Pharmacother. 2023. PMID: 36989711 Review.
-
Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs).Acta Biochim Pol. 2019 Mar 4;66(1):1-12. doi: 10.18388/abp.2018_2648. Acta Biochim Pol. 2019. PMID: 30831575 Review.
Cited by
-
Metabolic Program of Regulatory B Lymphocytes and Influence in the Control of Malignant and Autoimmune Situations.Front Immunol. 2021 Sep 28;12:735463. doi: 10.3389/fimmu.2021.735463. eCollection 2021. Front Immunol. 2021. PMID: 34650560 Free PMC article. Review.
-
Progress of research on the gut microbiome and its metabolite short-chain fatty acids in postmenopausal osteoporosis: a literature review.Front Med. 2025 Jun;19(3):474-492. doi: 10.1007/s11684-025-1129-3. Epub 2025 May 10. Front Med. 2025. PMID: 40347368 Review.
-
Short-chain fatty acids: key antiviral mediators of gut microbiota.Front Immunol. 2025 Jul 25;16:1614879. doi: 10.3389/fimmu.2025.1614879. eCollection 2025. Front Immunol. 2025. PMID: 40787446 Free PMC article. Review.
-
Evaluating the anti-inflammatory and antioxidant efficacy of complementary and alternative medicines (CAM) used for management of inflammatory bowel disease: a comprehensive review.Redox Rep. 2025 Dec;30(1):2471737. doi: 10.1080/13510002.2025.2471737. Epub 2025 Mar 8. Redox Rep. 2025. PMID: 40056427 Free PMC article. Review.
-
Health Benefits and Side Effects of Short-Chain Fatty Acids.Foods. 2022 Sep 15;11(18):2863. doi: 10.3390/foods11182863. Foods. 2022. PMID: 36140990 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

