A multicentre validation study of the diagnostic value of plasma neurofilament light
- PMID: 34099648
- PMCID: PMC8185001
- DOI: 10.1038/s41467-021-23620-z
A multicentre validation study of the diagnostic value of plasma neurofilament light
Abstract
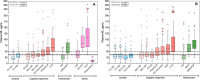
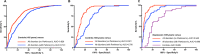
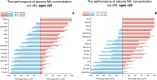
Increased cerebrospinal fluid neurofilament light (NfL) is a recognized biomarker for neurodegeneration that can also be assessed in blood. Here, we investigate plasma NfL as a marker of neurodegeneration in 13 neurodegenerative disorders, Down syndrome, depression and cognitively unimpaired controls from two multicenter cohorts: King's College London (n = 805) and the Swedish BioFINDER study (n = 1,464). Plasma NfL was significantly increased in all cortical neurodegenerative disorders, amyotrophic lateral sclerosis and atypical parkinsonian disorders. We demonstrate that plasma NfL is clinically useful in identifying atypical parkinsonian disorders in patients with parkinsonism, dementia in individuals with Down syndrome, dementia among psychiatric disorders, and frontotemporal dementia in patients with cognitive impairment. Data-driven cut-offs highlighted the fundamental importance of age-related clinical cut-offs for disorders with a younger age of onset. Finally, plasma NfL performs best when applied to indicate no underlying neurodegeneration, with low false positives, in all age-related cut-offs.
Conflict of interest statement
J.L. has received travel support and/or lecture honoraria from Biogen, Novartis, Merck, Roche, and Sanofi Genzyme; has served on scientific advisory boards for Biogen, Novartis, Merck, Alexion, Roche, and Sanofi Genzyme; serves on the editorial board of the Acta Neurologica Scandinavica; has received unconditional research grants from Biogen and Novartis. A.A.C. has served as a consultant or on advisory boards for Amylyx, Apellis, Biogen Idec, Brainstorm, Cytokinetics, GSK, Lilly, Mitsubishi Tanabe Pharma, Novartis, OrionPharma, Quralis, and Wave Pharmaceuticals. A.S. has been a consultant for AC-Immune and is a member of the scientific advisory board of ProMIS Neurosciences. P.S. has received speaker fees for Shire/Takeda and Sanofi Genzyme. H.Z. has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, and CogRx; has given lectures in symposia sponsored by Alzecure and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. K.B. has served as a consultant or at advisory boards for Alector, Alzheon, CogRx, Biogen, Lilly, Novartis, and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper. O.H. has acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals, and Euroimmun. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Biogen and Roche. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- MR/R024804/1/MRC_/Medical Research Council/United Kingdom
- P30 AG066507/AG/NIA NIH HHS/United States
- S011277/1/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- MR/L501529/1/MRC_/Medical Research Council/United Kingdom
- ALCHALABI-TALBOT/APR14/926-794/MNDA_/Motor Neurone Disease Association/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/R024901/1/MRC_/Medical Research Council/United Kingdom
- MR/S005145/1/MRC_/Medical Research Council/United Kingdom
- MR/S011277/1/MRC_/Medical Research Council/United Kingdom
- ALCHALABI-DOBSON/APR14/829-791/MNDA_/Motor Neurone Disease Association/United Kingdom
- G0600974/MRC_/Medical Research Council/United Kingdom
- 098330/Z/12/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

