SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care
- PMID: 34099652
- PMCID: PMC8184784
- DOI: 10.1038/s41467-021-23494-1
SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care
Abstract
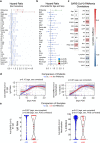
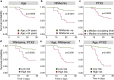
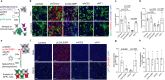
Prognostic characteristics inform risk stratification in intensive care unit (ICU) patients with coronavirus disease 2019 (COVID-19). We obtained blood samples (n = 474) from hospitalized COVID-19 patients (n = 123), non-COVID-19 ICU sepsis patients (n = 25) and healthy controls (n = 30). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA was detected in plasma or serum (RNAemia) of COVID-19 ICU patients when neutralizing antibody response was low. RNAemia is associated with higher 28-day ICU mortality (hazard ratio [HR], 1.84 [95% CI, 1.22-2.77] adjusted for age and sex). RNAemia is comparable in performance to the best protein predictors. Mannose binding lectin 2 and pentraxin-3 (PTX3), two activators of the complement pathway of the innate immune system, are positively associated with mortality. Machine learning identified 'Age, RNAemia' and 'Age, PTX3' as the best binary signatures associated with 28-day ICU mortality. In longitudinal comparisons, COVID-19 ICU patients have a distinct proteomic trajectory associated with mortality, with recovery of many liver-derived proteins indicating survival. Finally, proteins of the complement system and galectin-3-binding protein (LGALS3BP) are identified as interaction partners of SARS-CoV-2 spike glycoprotein. LGALS3BP overexpression inhibits spike-pseudoparticle uptake and spike-induced cell-cell fusion in vitro.
Conflict of interest statement
King’s College London has filed and licensed a patent application with regard to using PTX3 as a biomarker in sepsis. King’s College London has filed a patent application on the methods used to detect SARS-CoV-2 Spike protein-induced syncytia as described in this paper. A.C.H. is a board member and equity holder in ImmunoQure, A.G., and Gamma Delta Therapeutics, and is an equity holder in Adaptate Biotherapeutics.
Figures





References
-
- Vincent JL, et al. The S. O. F. A. (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. - DOI - PubMed
-
- Intensive Care National Audit And Research Centre. ICNARC Report on COVID-19 in Critical Care 31 July 2020. (2020).
Publication types
MeSH terms
Substances
Grants and funding
- CS-2016-16-011/DH_/Department of Health/United Kingdom
- MR/V040162/1/MRC_/Medical Research Council/United Kingdom
- RM/17/3/33381/BHF_/British Heart Foundation/United Kingdom
- RG/16/14/32397/BHF_/British Heart Foundation/United Kingdom
- CH/16/3/32406/BHF_/British Heart Foundation/United Kingdom
- FS/18/60/34181/BHF_/British Heart Foundation/United Kingdom
- FC001093/WT_/Wellcome Trust/United Kingdom
- RE/18/2/34213/BHF_/British Heart Foundation/United Kingdom
- MC/PC/15068/MRC_/Medical Research Council/United Kingdom
- FC001093 /WT_/Wellcome Trust/United Kingdom
- MR/R017751/1/MRC_/Medical Research Council/United Kingdom
- FS/19/58/34895/BHF_/British Heart Foundation/United Kingdom
- RG/19/11/34633/BHF_/British Heart Foundation/United Kingdom
- PG/17/48/32956/BHF_/British Heart Foundation/United Kingdom
- FS/17/65/33481/BHF_/British Heart Foundation/United Kingdom
- 106292/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_15068/MRC_/Medical Research Council/United Kingdom
- SP/17/10/33219/BHF_/British Heart Foundation/United Kingdom
- FC001093 /MRC_/Medical Research Council/United Kingdom
- CRUK_/Cancer Research UK/United Kingdom
- FC001093 /CRUK_/Cancer Research UK/United Kingdom
- CH/1999001/11735/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

