Genetic variation associated with thyroid autoimmunity shapes the systemic immune response to PD-1 checkpoint blockade
- PMID: 34099659
- PMCID: PMC8184890
- DOI: 10.1038/s41467-021-23661-4
Genetic variation associated with thyroid autoimmunity shapes the systemic immune response to PD-1 checkpoint blockade
Abstract
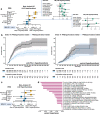
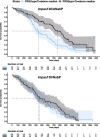
Activation of systemic immune responses using PD-1 checkpoint inhibitors is an essential approach to cancer therapy. Yet, the extent of benefit relative to risk of immune related adverse events (irAE) varies widely among patients. Here, we study endocrine irAE from 7 clinical trials across 6 cancers where atezolizumab (anti-PD-L1) was combined with chemotherapies and compared to standard of care. We show that atezolizumab-induced thyroid dysfunction is associated with longer survival. We construct a polygenic risk score (PRS) for lifetime risk of hypothyroidism using a GWAS from the UK Biobank and apply this PRS to genetic data collected from 2,616 patients of European ancestry from these trials. Patients with high PRS are at increased risk of atezolizumab-induced thyroid dysfunction and lower risk of death in triple negative breast cancer. Our results indicate that genetic variation associated with thyroid autoimmunity interacts with biological pathways driving the systemic immune response to PD-1 blockade.
Conflict of interest statement
All authors are current or former employees of Genentech/Roche. M.L.A. is currently an employee of insitro. Z.K. and G.S.C. are co-inventors on a patent application using the methods described here to identify patients treated with immune checkpoint inhibitors at high risk for endocrine irAE and selection of TNBC patients that might benefit from PD-1 checkpoint inhibitor treatment.
Figures


References
-
- Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer10.1038/s43018-020-0075-x (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

