Chemodivergent manganese-catalyzed C-H activation: modular synthesis of fluorogenic probes
- PMID: 34099672
- PMCID: PMC8185085
- DOI: 10.1038/s41467-021-23462-9
Chemodivergent manganese-catalyzed C-H activation: modular synthesis of fluorogenic probes
Abstract
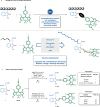
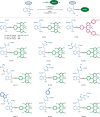
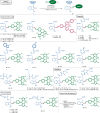
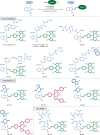
Bioorthogonal late-stage diversification of amino acids and peptides bears enormous potential for drug discovery and molecular imaging. Despite major accomplishments, these strategies largely rely on traditional, lengthy prefunctionalization methods, heavily involving precious transition-metal catalysis. Herein, we report on a resource-economical manganese(I)-catalyzed C-H fluorescent labeling of structurally complex peptides ensured by direct alkynylation and alkenylation manifolds. This modular strategy sets the stage for unraveling structure-activity relationships between structurally discrete fluorophores towards the rational design of BODIPY fluorogenic probes for real-time analysis of immune cell function.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Kuter K, et al. Adaptation within mitochondrial oxidative phosphorylation supercomplexes and membrane viscosity during degeneration of dopaminergic neurons in an animal model of early Parkinson’s disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2016;1862:741–753. doi: 10.1016/j.bbadis.2016.01.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

