An initial assessment of the involvement of transglutaminase2 in eosinophilic bronchitis using a disease model developed in C57BL/6 mice
- PMID: 34099759
- PMCID: PMC8184915
- DOI: 10.1038/s41598-021-90950-9
An initial assessment of the involvement of transglutaminase2 in eosinophilic bronchitis using a disease model developed in C57BL/6 mice
Abstract
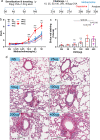
The detailed pathogenesis of eosinophilic bronchitis (EB) remains unclear. Transglutaminase 2 (TG2) has been implicated in many respiratory diseases including asthma. Herein, we aim to assess preliminarily the relationship of TG2 with EB in the context of the development of an appropriate EB model through ovalbumin (OVA) sensitization and challenge in the C57BL/6 mouse strain. Our data lead us to propose a 50 μg dose of OVA challenge as appropriate to establish an EB model in C57BL/6 mice, whereas a challenge with a 400 μg dose of OVA significantly induced asthma. Compared to controls, TG2 is up-regulated in the airway epithelium of EB mice and EB patients. When TG2 activity was inhibited by cystamine treatment, there were no effects on airway responsiveness; in contrast, the lung pathology score and eosinophil counts in bronchoalveolar lavage fluid were significantly increased whereas the cough frequency was significantly decreased. The expression levels of interleukin (IL)-4, IL-13, IL-6, mast cell protease7 and the transient receptor potential (TRP) ankyrin 1 (TRPA1), TRP vanilloid 1 (TRPV1) were significantly decreased. These data open the possibility of an involvement of TG2 in mediating the increased cough frequency in EB through the regulation of TRPA1 and TRPV1 expression. The establishment of an EB model in C57BL/6 mice opens the way for a genetic investigation of the involvement of TG2 and other molecules in this disease using KO mice, which are often generated in the C57BL/6 genetic background.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

