Optogenetic modulation of cardiac action potential properties may prevent arrhythmogenesis in short and long QT syndromes
- PMID: 34100384
- PMCID: PMC8262308
- DOI: 10.1172/jci.insight.147470
Optogenetic modulation of cardiac action potential properties may prevent arrhythmogenesis in short and long QT syndromes
Abstract
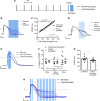
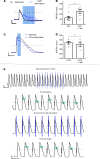
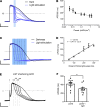
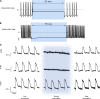
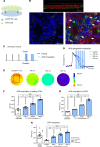
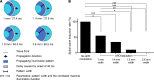
Abnormal action potential (AP) properties, as occurs in long or short QT syndromes (LQTS and SQTS, respectively), can cause life-threatening arrhythmias. Optogenetics strategies, utilizing light-sensitive proteins, have emerged as experimental platforms for cardiac pacing, resynchronization, and defibrillation. We tested the hypothesis that similar optogenetic tools can modulate the cardiomyocyte's AP properties, as a potentially novel antiarrhythmic strategy. Healthy control and LQTS/SQTS patient-specific human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were transduced to express the light-sensitive cationic channel channelrhodopsin-2 (ChR2) or the anionic-selective opsin, ACR2. Detailed patch-clamp, confocal-microscopy, and optical mapping studies evaluated the ability of spatiotemporally defined optogenetic protocols to modulate AP properties and prevent arrhythmogenesis in the hiPSC-CMs cell/tissue models. Depending on illumination timing, light-induced ChR2 activation induced robust prolongation or mild shortening of AP duration (APD), while ACR2 activation allowed effective APD shortening. Fine-tuning these approaches allowed for the normalization of pathological AP properties and suppression of arrhythmogenicity in the LQTS/SQTS hiPSC-CM cellular models. We next established a SQTS-hiPSC-CMs-based tissue model of reentrant-arrhythmias using optogenetic cross-field stimulation. An APD-modulating optogenetic protocol was then designed to dynamically prolong APD of the propagating wavefront, completely preventing arrhythmogenesis in this model. This work highlights the potential of optogenetics in studying repolarization abnormalities and in developing novel antiarrhythmic therapies.
Keywords: Arrhythmias; Cardiology; Gene therapy; Stem cells; iPS cells.
Conflict of interest statement
Figures


Similar articles
-
Modeling Reentry in the Short QT Syndrome With Human-Induced Pluripotent Stem Cell-Derived Cardiac Cell Sheets.J Am Coll Cardiol. 2019 May 14;73(18):2310-2324. doi: 10.1016/j.jacc.2019.02.055. J Am Coll Cardiol. 2019. PMID: 31072576
-
Optogenetic Control of Human Induced Pluripotent Stem Cell-Derived Cardiac Tissue Models.J Am Heart Assoc. 2022 Feb 15;11(4):e021615. doi: 10.1161/JAHA.121.021615. Epub 2022 Feb 3. J Am Heart Assoc. 2022. PMID: 35112880 Free PMC article.
-
Gene- and variant-specific efficacy of serum/glucocorticoid-regulated kinase 1 inhibition in long QT syndrome types 1 and 2.Europace. 2023 May 19;25(5):euad094. doi: 10.1093/europace/euad094. Europace. 2023. PMID: 37099628 Free PMC article.
-
Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as Models for Cardiac Channelopathies: A Primer for Non-Electrophysiologists.Circ Res. 2018 Jul 6;123(2):224-243. doi: 10.1161/CIRCRESAHA.118.311209. Circ Res. 2018. PMID: 29976690 Free PMC article. Review.
-
Principles of Optogenetic Methods and Their Application to Cardiac Experimental Systems.Front Physiol. 2019 Sep 11;10:1096. doi: 10.3389/fphys.2019.01096. eCollection 2019. Front Physiol. 2019. PMID: 31572204 Free PMC article. Review.
Cited by
-
Opto-electronic feedback control of membrane potential for real-time control of action potentials.Cell Rep Methods. 2023 Dec 18;3(12):100671. doi: 10.1016/j.crmeth.2023.100671. Epub 2023 Dec 11. Cell Rep Methods. 2023. PMID: 38086387 Free PMC article.
-
A micro-LED array based platform for spatio-temporal optogenetic control of various cardiac models.Sci Rep. 2023 Nov 9;13(1):19490. doi: 10.1038/s41598-023-46149-1. Sci Rep. 2023. PMID: 37945622 Free PMC article.
-
Primary Electrical Heart Disease-Principles of Pathophysiology and Genetics.Int J Mol Sci. 2024 Feb 2;25(3):1826. doi: 10.3390/ijms25031826. Int J Mol Sci. 2024. PMID: 38339103 Free PMC article. Review.
-
Optogenetically mediated large volume suppression and synchronized excitation of human ventricular cardiomyocytes.Pflugers Arch. 2023 Dec;475(12):1479-1503. doi: 10.1007/s00424-023-02831-x. Epub 2023 Jul 7. Pflugers Arch. 2023. PMID: 37415050
-
Induced Pluripotent Stem Cells in Congenital Long QT Syndrome: Research Progress and Clinical Applications.Rev Cardiovasc Med. 2025 Apr 22;26(4):28251. doi: 10.31083/RCM28251. eCollection 2025 Apr. Rev Cardiovasc Med. 2025. PMID: 40351699 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

