Scale-up economics for cultured meat
- PMID: 34101164
- PMCID: PMC8362201
- DOI: 10.1002/bit.27848
Scale-up economics for cultured meat
Abstract
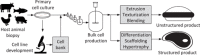
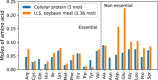
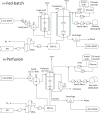
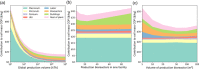
This analysis examines the potential of "cultured meat" products made from edible animal cell culture to measurably displace the global consumption of conventional meat. Recognizing that the scalability of such products must in turn depend on the scale and process intensity of animal cell production, this study draws on technoeconomic analysis perspectives in industrial fermentation and upstream biopharmaceuticals to assess the extent to which animal cell culture could be scaled like a fermentation process. Low growth rate, metabolic inefficiency, catabolite inhibition, and shear-induced cell damage will all limit practical bioreactor volume and attainable cell density. Equipment and facilities with adequate microbial contamination safeguards have high capital costs. The projected costs of suitably pure amino acids and protein growth factors are also high. The replacement of amino-acid media with plant protein hydrolysates is discussed and requires further study. Capital- and operating-cost analyses of conceptual cell-mass production facilities indicate economics that would likely preclude the affordability of their products as food. The analysis concludes that metabolic efficiency enhancements and the development of low-cost media from plant hydrolysates are both necessary but insufficient conditions for displacement of conventional meat by cultured meat.
Keywords: animal cell culture; bioreactor design; fermentation; techno-economic analysis.
© 2021 The Authors. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Figures




References
-
- Alberts, B., (Ed.). (2002). Molecular biology of the cell (4th ed). Garland Science.
-
- ASME . (2016). Bioprocessing equipment (BPE‐2016).
-
- Babcock, J., Smith, S., Huttinga, H., & Merrill, D. (2007). Enhancing performance in cell culture. Genetic Engineering and Biotechnology News, 27(20), https://www.genengnews.com/magazine/81/enhancing-performance-in-cell-cul...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

