Moving Pharmacogenetics Into Practice: It's All About the Evidence!
- PMID: 34101169
- PMCID: PMC8376790
- DOI: 10.1002/cpt.2327
Moving Pharmacogenetics Into Practice: It's All About the Evidence!
Abstract
The evidence for pharmacogenetics has grown rapidly in recent decades. However, the strength of evidence required for the clinical implementation of pharmacogenetics is highly debated. Therefore, the purpose of this review is to summarize different perspectives on the evidence required for the clinical implementation of pharmacogenetics. First, we present two patient cases that demonstrate how knowledge of pharmacogenetic evidence affected their care. Then we summarize resources that curate pharmacogenetic evidence, types of evidence (with an emphasis on randomized controlled trials [RCT]) and their limitations, and different perspectives from implementers, clinicians, and patients. We compare pharmacogenetics to a historical example (i.e., the evidence required for the clinical implementation of pharmacokinetics/therapeutic drug monitoring), and we provide future perspectives on the evidence for pharmacogenetic panels and the need for more education in addition to evidence. Although there are differences in the interpretation of pharmacogenetic evidence across resources, efforts for standardization are underway. Survey data illustrate the value of pharmacogenetic testing from the patient perspective, with their providers seen as key to ensuring maximum benefit from test results. However, clinicians and practice guidelines from medical societies often rely on RCT data to guide treatment decisions, which are not always feasible or ethical in pharmacogenetics. Thus, recognition of other types of evidence to support pharmacogenetic implementation is needed. Among pharmacogenetic implementers, consistent evidence of pharmacogenetic associations is deemed most critical. Ultimately, moving pharmacogenetics into practice will require consideration of multiple stakeholder perspectives, keeping particularly attuned to the voice of the ultimate stakeholder-the patient.
© 2021 The Authors. Clinical Pharmacology & Therapeutics © 2021 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Figures
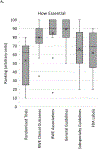




References
-
- Hicks JK et al.Implementation of Clinical Pharmacogenomics within a Large Health System: From Electronic Health Record Decision Support to Consultation Services. Pharmacotherapy 36, 940–8 (2016). - PubMed
-
- van der Wouden CH et al.Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin Pharmacol Ther 101, 341–58 (2017). - PubMed
-
- Petry N et al.Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics 20, 903–13 (2019). - PubMed

