A Chlamydial Plasmid-Dependent Secretion System for the Delivery of Virulence Factors to the Host Cytosol
- PMID: 34101486
- PMCID: PMC8262877
- DOI: 10.1128/mBio.01179-21
A Chlamydial Plasmid-Dependent Secretion System for the Delivery of Virulence Factors to the Host Cytosol
Abstract
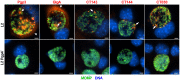
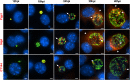
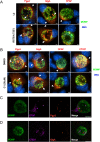
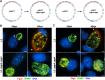
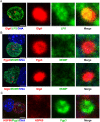
Chlamydia are obligate intracellular Gram-negative bacteria distinguished by a unique developmental biology confined within a parasitophorous vacuole termed an inclusion. The chlamydial plasmid is a central virulence factor in the pathogenesis of infection. Plasmid gene protein 4 (Pgp4) regulates the expression of plasmid gene protein 3 (Pgp3) and chromosomal glycogen synthase (GlgA), virulence factors secreted from the inclusion to the host cytosol by an unknown mechanism. Here, we identified a plasmid-dependent secretion system for the cytosolic delivery of Pgp3 and GlgA. The secretion system consisted of a segregated population of globular structures originating from midcycle reticulate bodies. Globular structures contained the Pgp4-regulated proteins CT143, CT144, and CT050 in addition to Pgp3 and GlgA. Genetic replacement of Pgp4 with Pgp3 or GlgA negated the formation of globular structures, resulting in retention of Pgp3 and GlgA in chlamydial organisms. The generation of globular structures and secretion of virulence factors occurred independently of type 2 and type 3 secretion systems. Globular structures were enriched with lipopolysaccharide but lacked detectable major outer membrane protein and heat shock protein 60, implicating them as outer membrane vesicles. Thus, we have discovered a novel chlamydial plasmid-dependent secretion system that transports virulence factor cargo from the chlamydial inclusion to the host cytosol. IMPORTANCE The Chlamydia trachomatis plasmid regulates the expression and secretion of immune evasion virulence factors to the host cytosol by an unknown mechanism. In this study, we identified a novel plasmid gene protein 4 (Pgp4)-dependent secretion system. The system consists of globular structures distinct from typical chlamydial developmental forms that export Pgp3 and GlgA to the host cytosol. Globular structures emerged at mid-chlamydial growth cycle from distinct populations of reticulate bodies. The formation of globular structures occurred independently of known chlamydial secretion systems. These results identify a Pgp4-dependent secretory system required for exporting plasmid regulated virulence factors to the host cytosol.
Keywords: Chlamydia; plasmid; secretion; virulence factors.
Figures


References
-
- World Health Organization. 2016. WHO Guidelines for the Treatment of Chlamydia trachomatis. World Health Organization, Geneva, Switzerland. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
